Upregulated integrin α11 in the stroma of cutaneous squamous cell carcinoma promotes skin carcinogenesis
- PMID: 36003785
- PMCID: PMC9393502
- DOI: 10.3389/fonc.2022.981009
Upregulated integrin α11 in the stroma of cutaneous squamous cell carcinoma promotes skin carcinogenesis
Abstract
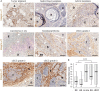
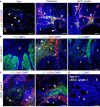
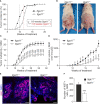
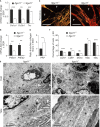
Integrin α11β1 is a collagen-binding integrin that is needed to induce and maintain the myofibroblast phenotype in fibrotic tissues and during wound healing. The expression of the α11 is upregulated in cancer-associated fibroblasts (CAFs) in various human neoplasms. We investigated α11 expression in human cutaneous squamous cell carcinoma (cSCC) and in benign and premalignant human skin lesions and monitored its effects on cSCC development by subjecting α11-knockout (Itga11-/- ) mice to the DMBA/TPA skin carcinogenesis protocol. α11-deficient mice showed significantly decreased tumor cell proliferation, leading to delayed tumor development and reduced tumor burden. Integrin α11 expression was significantly upregulated in the desmoplastic tumor stroma of human and mouse cSCCs, and the highest α11 expression was detected in high-grade tumors. Our results point to a reduced ability of α11-deficient stromal cells to differentiate into matrix-producing and tumor-promoting CAFs and suggest that this is one causative mechanism underlying the observed decreased tumor growth. An unexpected finding in our study was that, despite reduced CAF activation, the α11-deficient skin tumors were characterized by the presence of thick and regularly aligned collagen bundles. This finding was attributed to a higher expression of TGFβ1 and collagen crosslinking lysyl oxidases in the Itga11-/- tumor stroma. In summary, our data suggest that α11β1 operates in a complex interactive tumor environment to regulate ECM synthesis and collagen organization and thus foster cSCC growth. Further studies with advanced experimental models are still needed to define the exact roles and molecular mechanisms of stromal α11β1 in skin tumorigenesis.
Keywords: DMBA/TPA; cancer-associated fibroblast; collagen; extracellular matrix; lysyl oxidase; myofibroblast; tumor microenvironment.
Copyright © 2022 Martínez-Nieto, Teppo, Petrelius, Izzi, Devarajan, Petäistö, Liu, Kim, Karppinen, Ruotsalainen, Koivunen, Mäki, Walker, Pihlajaniemi, Gullberg and Heljasvaara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Stromal integrin α11-deficiency reduces interstitial fluid pressure and perturbs collagen structure in triple-negative breast xenograft tumors.BMC Cancer. 2019 Mar 15;19(1):234. doi: 10.1186/s12885-019-5449-z. BMC Cancer. 2019. PMID: 30876468 Free PMC article.
-
α11β1 Integrin is Induced in a Subset of Cancer-Associated Fibroblasts in Desmoplastic Tumor Stroma and Mediates In Vitro Cell Migration.Cancers (Basel). 2019 Jun 1;11(6):765. doi: 10.3390/cancers11060765. Cancers (Basel). 2019. PMID: 31159419 Free PMC article.
-
Integrin α11β1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer.Oncogene. 2016 Apr 14;35(15):1899-908. doi: 10.1038/onc.2015.254. Epub 2015 Jul 6. Oncogene. 2016. PMID: 26148229 Free PMC article.
-
Integrin α11β1: a major collagen receptor on fibroblastic cells.Adv Exp Med Biol. 2014;819:73-83. doi: 10.1007/978-94-017-9153-3_5. Adv Exp Med Biol. 2014. PMID: 25023168 Review.
-
New perspectives on role of tumor microenvironment in progression of cutaneous squamous cell carcinoma.Cell Tissue Res. 2016 Sep;365(3):691-702. doi: 10.1007/s00441-016-2457-z. Epub 2016 Jul 14. Cell Tissue Res. 2016. PMID: 27411692 Review.
Cited by
-
ITGA11, a Prognostic Factor Associated with Immunity in Gastric Adenocarcinoma.Int J Gen Med. 2024 Feb 5;17:471-483. doi: 10.2147/IJGM.S444786. eCollection 2024. Int J Gen Med. 2024. PMID: 38344679 Free PMC article.
-
CHD1L accelated the progression of cutaneous squamous cell carcinoma via promoting PI3K/PD-L1 signaling pathway induced M2 polarization of TAMs.Sci Rep. 2024 Dec 28;14(1):31231. doi: 10.1038/s41598-024-82594-2. Sci Rep. 2024. PMID: 39732937 Free PMC article.
-
The Mutational and Microenvironmental Landscape of Cutaneous Squamous Cell Carcinoma: A Review.Cancers (Basel). 2024 Aug 21;16(16):2904. doi: 10.3390/cancers16162904. Cancers (Basel). 2024. PMID: 39199674 Free PMC article. Review.
-
TDO2 + cancer-associated fibroblasts mediate cutaneous squamous cell carcinoma immune escape via impeding infiltration of CD8 + T cells.Cancer Immunol Immunother. 2025 Jan 3;74(2):67. doi: 10.1007/s00262-024-03921-0. Cancer Immunol Immunother. 2025. PMID: 39751882 Free PMC article.
-
Cancer-associated fibroblast cell surface markers as potential biomarkers or therapeutic targets in lung cancer.Cancer Drug Resist. 2024 Sep 10;7:32. doi: 10.20517/cdr.2024.55. eCollection 2024. Cancer Drug Resist. 2024. PMID: 39403603 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

