Expanding the chemical diversity of M13 bacteriophage
- PMID: 36003937
- PMCID: PMC9393631
- DOI: 10.3389/fmicb.2022.961093
Expanding the chemical diversity of M13 bacteriophage
Abstract
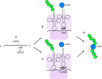
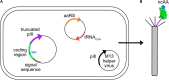
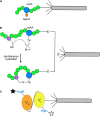
Bacteriophage M13 virions are very stable nanoparticles that can be modified by chemical and genetic methods. The capsid proteins can be functionalized in a variety of chemical reactions without loss of particle integrity. In addition, Genetic Code Expansion (GCE) permits the introduction of non-canonical amino acids (ncAAs) into displayed peptides and proteins. The incorporation of ncAAs into phage libraries has led to the discovery of high-affinity binders with low nanomolar dissociation constant (K D) values that can potentially serve as inhibitors. This article reviews how bioconjugation and the incorporation of ncAAs during translation have expanded the chemistry of peptides and proteins displayed by M13 virions for a variety of purposes.
Keywords: antibody fragments; bioconjugation; combinatorial peptide libraries; cross-linking; cyclization; peptide; phage-display; stop codon suppression.
Copyright © 2022 Allen, Grahn, Kourentzi, Willson, Waldrop, Guo and Kay.
Conflict of interest statement
GA, AG, and BK were employed of a biotech start-up company (Tango Biosciences, Inc.). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

