On the Oxidative Valorization of Lignin to High-Value Chemicals: A Critical Review of Opportunities and Challenges
- PMID: 36004569
- PMCID: PMC9825943
- DOI: 10.1002/cssc.202201232
On the Oxidative Valorization of Lignin to High-Value Chemicals: A Critical Review of Opportunities and Challenges
Abstract
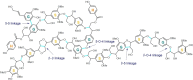
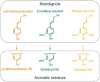
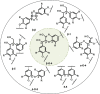
The efficient valorization of lignin is crucial if we are to replace current petroleum-based feedstock and establish more sustainable and competitive lignocellulosic biorefineries. Pulp and paper mills and second-generation biorefineries produce large quantities of low-value technical lignin as a by-product, which is often combusted on-site for energy recovery. This Review focuses on the conversion of technical lignins by oxidative depolymerization employing heterogeneous catalysts. It scrutinizes the current literature describing the use of various heterogeneous catalysts in the oxidative depolymerization of lignin and includes a comparison of the methods, catalyst loadings, reaction media, and types of catalyst applied, as well as the reaction products and yields. Furthermore, current techniques for the determination of product yields and product recovery are discussed. Finally, challenges and suggestions for future approaches are outlined.
Keywords: analytical methods; biomass; heterogeneous catalysis; lignin valorization; oxidative depolymerization.
© 2022 The Authors. ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
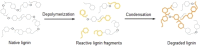

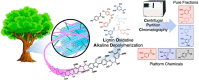
References
-
- Bruijnincx P. C. A., Rinaldi R., Weckhuysen B. M., Green Chem. 2015, 17, 4860–4861.
-
- Schutyser W., Renders T., Van den Bosch S., Koelewijn S.-F., Beckham G. T., Sels B. F., Chem. Soc. Rev. 2018, 47, 852–908. - PubMed
-
- Lahive C. W., Deuss P. J., Lancefield C. S., Sun Z., Cordes D. B., Young C. M., Tran F., Slawin A. M. Z., de Vries J. G., Kamer P. C. J., Westwood N. J., Barta K., J. Am. Chem. Soc. 2016, 138, 8900–8911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

