Plasma Cell-Free DNA Predicts Survival and Maps Specific Sources of Injury in Pulmonary Arterial Hypertension
- PMID: 36004627
- PMCID: PMC9529801
- DOI: 10.1161/CIRCULATIONAHA.121.056719
Plasma Cell-Free DNA Predicts Survival and Maps Specific Sources of Injury in Pulmonary Arterial Hypertension
Abstract
Background: Cell-free DNA (cfDNA) is a noninvasive marker of cellular injury. Its significance in pulmonary arterial hypertension (PAH) is unknown.
Methods: Plasma cfDNA was measured in 2 PAH cohorts (A, n=48; B, n=161) and controls (n=48). Data were collected for REVEAL 2.0 (Registry to Evaluate Early and Long-Term PAH Disease Management) scores and outcome determinations. Patients were divided into the following REVEAL risk groups: low (≤6), medium (7-8), and high (≥9). Total cfDNA concentrations were compared among controls and PAH risk groups by 1-way analysis of variance. Log-rank tests compared survival between cfDNA tertiles and REVEAL risk groups. Areas under the receiver operating characteristic curve were estimated from logistic regression models. A sample subset from cohort B (n=96) and controls (n=16) underwent bisulfite sequencing followed by a deconvolution algorithm to map cell-specific cfDNA methylation patterns, with concentrations compared using t tests.
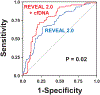
Results: In cohort A, median (interquartile range) age was 62 years (47-71), with 75% female, and median (interquartile range) REVEAL 2.0 was 6 (4-9). In cohort B, median (interquartile range) age was 59 years (49-71), with 69% female, and median (interquartile range) REVEAL 2.0 was 7 (6-9). In both cohorts, cfDNA concentrations differed among patients with PAH of varying REVEAL risk and controls (analysis of variance P≤0.002) and were greater in the high-risk compared with the low-risk category (P≤0.002). In cohort B, death or lung transplant occurred in 14 of 54, 23 of 53, and 35 of 54 patients in the lowest, middle, and highest cfDNA tertiles, respectively. cfDNA levels stratified as tertiles (log-rank: P=0.0001) and REVEAL risk groups (log-rank: P<0.0001) each predicted transplant-free survival. The addition of cfDNA to REVEAL improved discrimination (area under the receiver operating characteristic curve, 0.72-0.78; P=0.02). Compared with controls, methylation analysis in patients with PAH revealed increased cfDNA originating from erythrocyte progenitors, neutrophils, monocytes, adipocytes, natural killer cells, vascular endothelium, and cardiac myocytes (Bonferroni adjusted P<0.05). cfDNA concentrations derived from erythrocyte progenitor cells, cardiac myocytes, and vascular endothelium were greater in patients with PAH with high-risk versus low-risk REVEAL scores (P≤0.02).
Conclusions: Circulating cfDNA is elevated in patients with PAH, correlates with disease severity, and predicts worse survival. Results from cfDNA methylation analyses in patients with PAH are consistent with prevailing paradigms of disease pathogenesis.
Keywords: biomarkers; cell-free nucleic acids; endothelium, vascular; methylation; monocytes; myocytes, cardiac; pulmonary arterial hypertension; risk assessment.
Figures





References
-
- Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. - PubMed
-
- Nagaya N, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Nakanishi N, Yamagishi M, Kunieda T and Miyatake K. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med. 1999;160:487–92. - PubMed
-
- Torbicki A, Kurzyna M, Kuca P, Fijalkowska A, Sikora J, Florczyk M, Pruszczyk P, Burakowski J and Wawrzynska L. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844–8. - PubMed
-
- Quarck R, Nawrot T, Meyns B and Delcroix M. C-reactive protein: a new predictor of adverse outcome in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;53:1211–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

