Butyrylcholinesterase distribution in the mouse gastrointestinal tract: An immunohistochemical study
- PMID: 36004682
- PMCID: PMC9877478
- DOI: 10.1111/joa.13754
Butyrylcholinesterase distribution in the mouse gastrointestinal tract: An immunohistochemical study
Abstract
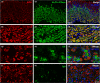
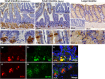
Butyrylcholinesterase (BChE) is a hydrolytic enzyme that together with acetylcholinesterase (AChE) belongs to the cholinesterase family. Whereas AChE has a well-established role in regulating cholinergic neurotransmission in central and peripheral synapses, the physiological role of BChE remains elusive. In this morphological immunohistochemical and double-label confocal microscopy study we investigated the distribution of BChE in the mouse gastrointestinal tract. BChE-positive cells were detected in the liver (both in hepatocytes and cholangiocytes), in the keratinised layers of the squamous epithelium of the oesophagus and forestomach, in the oxyntic mucosa of the stomach, in the mucus-secreting cells of duodenal Brunner glands and the small and large intestinal mucosa. Interestingly, BChE-positive cells were often detected close to gastrointestinal proliferative niches. In the oxyntic mucosa, the close proximity of ghrelin-producing and BChE-positive parietal cells suggests that BChE may be involved in ghrelin hydrolysation through paracrine action. To our knowledge, this is the first comprehensive morphological study performed to gain insight into the physiological role of BChE in the gastrointestinal tract.
Keywords: Ki67; Paneth cells; acetylcholine; acetylcholinesterase; ghrelin; non-neuronal cholinergic system; parietal cells.
© 2022 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Barker, N. , Huch, M. , Kujala, P. , van de Wetering, M. , Snippert, H.J. , van Es, J.H. et al. (2010) Lgr5(+ve) stem cells drive self‐renewal in the stomach and build long‐lived gastric units in vitro. Cell Stem Cell, 6, 25–36. - PubMed
-
- Boass, A. & Wilson, T.H. (1964) Cellular localization of gastric intrinsic factor in the rat. American Journal of Physiology, 206, 78–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

