Enantioselective Total Synthesis of (-)-Limaspermidine and (-)-Kopsinine by a Nitroaryl Transfer Cascade Strategy
- PMID: 36004723
- PMCID: PMC9826323
- DOI: 10.1002/anie.202210592
Enantioselective Total Synthesis of (-)-Limaspermidine and (-)-Kopsinine by a Nitroaryl Transfer Cascade Strategy
Abstract
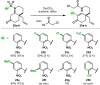
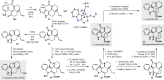
We report an intramolecular conjugate addition/Truce-Smiles/E1cb cascade of 2-nitrobenzenesulfonamide-functionalized cyclohexenones as a new entry to the core scaffold of monoterpene indole alkaloids. The method was applied to the asymmetric total synthesis of (-)-limaspermidine, (-)-kopsinilam, and (-)-kopsinine, as well as the framework of the kopsifoline alkaloids, thus highlighting its complementarity to existing approaches involving the use of indole-based starting materials or the interrupted Fischer indole synthesis. Furthermore, we show that the cascade tolerates various substituents on the nitroarene, opening the way to other natural products as well as non-natural analogues.
Keywords: Alkaloids; Asymmetric Synthesis; Domino Reactions; Total Synthesis.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Woodward R. B., Cava M. P., Ollis W. D., Hunger A., Daeniker H. U., Schenker K., J. Am. Chem. Soc. 1954, 76, 4749–4751.
-
- Medina J. D., Di Genova L., Planta Med. 1979, 37, 165–167.
-
- Ban Y., Honma Y., Ohnuma T., Heterocycles 1976, 5, 47–51.
-
- Racemic syntheses:
-
- Overman L. E., Robertson G. M., Robichaud A. J., J. Am. Chem. Soc. 1991, 113, 2598–2610;
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

