A multi-model approach to predict efficacious clinical dose for an anti-TGF-β antibody (GC2008) in the treatment of osteogenesis imperfecta
- PMID: 36004727
- PMCID: PMC9662198
- DOI: 10.1002/psp4.12857
A multi-model approach to predict efficacious clinical dose for an anti-TGF-β antibody (GC2008) in the treatment of osteogenesis imperfecta
Abstract
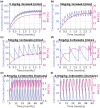
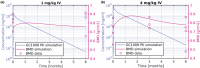
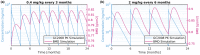
Osteogenesis imperfecta (OI) is a heterogeneous group of inherited bone dysplasias characterized by reduced skeletal mass and bone fragility. Although the primary manifestation of the disease involves the skeleton, OI is a generalized connective tissue disorder that requires a multidisciplinary treatment approach. Recent studies indicate that application of a transforming growth factor beta (TGF-β) neutralizing antibody increased bone volume fraction (BVF) and strength in an OI mouse model and improved bone mineral density (BMD) in a small cohort of patients with OI. In this work, we have developed a multitiered quantitative pharmacology approach to predict human efficacious dose of a new anti-TGF-β antibody drug candidate (GC2008). This method aims to translate GC2008 pharmacokinetic/pharmacodynamic (PK/PD) relationship in patients, using a number of appropriate mathematical models and available preclinical and clinical data. Compartmental PK linked with an indirect PD effect model was used to characterize both pre-clinical and clinical PK/PD data and predict a GC2008 dose that would significantly increase BMD or BVF in patients with OI. Furthermore, a physiologically-based pharmacokinetic model incorporating GC2008 and the body's physiological properties was developed and used to predict a GC2008 dose that would decrease the TGF-β level in bone to that of healthy individuals. By using multiple models, we aim to reveal information for different aspects of OI disease that will ultimately lead to a more informed dose projection of GC2008 in humans. The different modeling efforts predicted a similar range of pharmacologically relevant doses in patients with OI providing an informed approach for an early clinical dose setting.
© 2022 Sanofi. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures


Similar articles
-
Effect of Anti-TGF-β Treatment in a Mouse Model of Severe Osteogenesis Imperfecta.J Bone Miner Res. 2019 Feb;34(2):207-214. doi: 10.1002/jbmr.3617. Epub 2018 Nov 29. J Bone Miner Res. 2019. PMID: 30357929
-
Targeting TGF-β for treatment of osteogenesis imperfecta.J Clin Invest. 2022 Apr 1;132(7):e152571. doi: 10.1172/JCI152571. J Clin Invest. 2022. PMID: 35113812 Free PMC article. Clinical Trial.
-
Correlations Between Bone Mechanical Properties and Bone Composition Parameters in Mouse Models of Dominant and Recessive Osteogenesis Imperfecta and the Response to Anti-TGF-β Treatment.J Bone Miner Res. 2017 Feb;32(2):347-359. doi: 10.1002/jbmr.2997. Epub 2016 Oct 20. J Bone Miner Res. 2017. PMID: 27649409 Free PMC article.
-
Mortality and morbidity in patients with osteogenesis imperfecta in Denmark.Dan Med J. 2018 Apr;65(4):B5454. Dan Med J. 2018. PMID: 29619932 Review.
-
Osteogenesis Imperfecta: Muscle-Bone Interactions when Bi-directionally Compromised.Curr Osteoporos Rep. 2018 Aug;16(4):478-489. doi: 10.1007/s11914-018-0456-6. Curr Osteoporos Rep. 2018. PMID: 29909596 Review.
Cited by
-
Application of machine learning in combination with mechanistic modeling to predict plasma exposure of small molecules.Front Syst Biol. 2023 Jun 20;3:1180948. doi: 10.3389/fsysb.2023.1180948. eCollection 2023. Front Syst Biol. 2023. PMID: 40809487 Free PMC article.
-
Machine learning framework to predict pharmacokinetic profile of small molecule drugs based on chemical structure.Clin Transl Sci. 2024 May;17(5):e13824. doi: 10.1111/cts.13824. Clin Transl Sci. 2024. PMID: 38752574 Free PMC article.
References
-
- Marini JC, Forlino A, Bachinger HP, et al. Osteogenesis imperfecta. Nat Rev Dis Primers. 2017;3:17052. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

