ATP:Mg2+ shapes material properties of protein-RNA condensates and their partitioning of clients
- PMID: 36004782
- PMCID: PMC9674983
- DOI: 10.1016/j.bpj.2022.08.025
ATP:Mg2+ shapes material properties of protein-RNA condensates and their partitioning of clients
Abstract
Many cellular condensates are heterotypic mixtures of proteins and RNA formed in complex environments. Magnesium ions (Mg2+) and ATP can impact RNA folding, and local intracellular levels of these factors can vary significantly. However, the effect of ATP:Mg2+ on the material properties of protein-RNA condensates is largely unknown. Here, we use an in vitro condensate model of nucleoli, made from nucleophosmin 1 (NPM1) proteins and ribosomal RNA (rRNA), to study the effect of ATP:Mg2+. While NPM1 dynamics remain unchanged at increasing Mg2+ concentrations, the internal RNA dynamics dramatically slowed until a critical point, where gel-like states appeared, suggesting the RNA component alone forms a viscoelastic network that undergoes maturation driven by weak multivalent interactions. ATP reverses this arrest and liquefies the gel-like structures. ATP:Mg2+ also influenced the NPM1-rRNA composition of condensates and enhanced the partitioning of two clients: an arginine-rich peptide and a small nuclear RNA. By contrast, larger ribosome partitioning shows dependence on ATP:Mg2+ and can become reversibly trapped around NPM1-rRNA condensates. Lastly, we show that dissipative enzymatic reactions that deplete ATP can be used to control the shape, composition, and function of condensates. Our results illustrate how intracellular environments may regulate the state and client partitioning of RNA-containing condensates.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no conflict of interest.
Figures
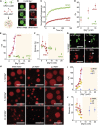


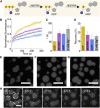
Comment in
-
Small molecules playing big roles: Tuning material properties of nucleolar condensates.Biophys J. 2022 Oct 18;121(20):3768-3770. doi: 10.1016/j.bpj.2022.08.043. Epub 2022 Sep 2. Biophys J. 2022. PMID: 36108626 Free PMC article. No abstract available.
Similar articles
-
Crowding-induced phase separation and gelling by co-condensation of PEG in NPM1-rRNA condensates.Biophys J. 2023 Jan 17;122(2):397-407. doi: 10.1016/j.bpj.2022.12.001. Epub 2022 Dec 5. Biophys J. 2023. PMID: 36463407 Free PMC article.
-
ZNF692 regulates nucleolar morphology by interacting with NPM1 and modifying its self-assembly properties.J Biol Chem. 2024 Mar;300(3):105773. doi: 10.1016/j.jbc.2024.105773. Epub 2024 Feb 19. J Biol Chem. 2024. PMID: 38382671 Free PMC article.
-
C9orf72 Poly(PR) Dipeptide Repeats Disturb Biomolecular Phase Separation and Disrupt Nucleolar Function.Mol Cell. 2019 May 16;74(4):713-728.e6. doi: 10.1016/j.molcel.2019.03.019. Epub 2019 Apr 10. Mol Cell. 2019. PMID: 30981631 Free PMC article.
-
Using quantitative reconstitution to investigate multicomponent condensates.RNA. 2022 Jan;28(1):27-35. doi: 10.1261/rna.079008.121. Epub 2021 Nov 12. RNA. 2022. PMID: 34772789 Free PMC article. Review.
-
Nuclear Protein Condensates and Their Properties in Regulation of Gene Expression.J Mol Biol. 2022 Jan 15;434(1):167151. doi: 10.1016/j.jmb.2021.167151. Epub 2021 Jul 14. J Mol Biol. 2022. PMID: 34271007 Free PMC article. Review.
Cited by
-
The mechanobiology of biomolecular condensates.Biophys Rev (Melville). 2025 Mar 25;6(1):011310. doi: 10.1063/5.0236610. eCollection 2025 Mar. Biophys Rev (Melville). 2025. PMID: 40160200 Free PMC article. Review.
-
Chaperone-mediated heterotypic phase separation regulates liquid-to-solid phase transitions of tau into amyloid fibrils.Sci Adv. 2025 Jun 6;11(23):eads1241. doi: 10.1126/sciadv.ads1241. Epub 2025 Jun 6. Sci Adv. 2025. PMID: 40479073 Free PMC article.
-
Adaptive ATP-induced molecular condensation in membranized protocells.Proc Natl Acad Sci U S A. 2025 Apr;122(13):e2419507122. doi: 10.1073/pnas.2419507122. Epub 2025 Mar 24. Proc Natl Acad Sci U S A. 2025. PMID: 40127264
-
Small molecules playing big roles: Tuning material properties of nucleolar condensates.Biophys J. 2022 Oct 18;121(20):3768-3770. doi: 10.1016/j.bpj.2022.08.043. Epub 2022 Sep 2. Biophys J. 2022. PMID: 36108626 Free PMC article. No abstract available.
-
HP1 loses its chromatin clustering and phase separation function across evolution.Nat Commun. 2025 Jul 10;16(1):6375. doi: 10.1038/s41467-025-61749-3. Nat Commun. 2025. PMID: 40640210 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

