Effects of Ceftriaxone on Oxidative Stress and Inflammation in a Rat Model of Chronic Cerebral Hypoperfusion
- PMID: 36004858
- PMCID: PMC9404883
- DOI: 10.3390/bs12080287
Effects of Ceftriaxone on Oxidative Stress and Inflammation in a Rat Model of Chronic Cerebral Hypoperfusion
Abstract
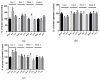
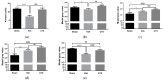
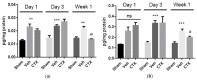
Ceftriaxone (CTX) exerts a neuroprotective effect by decreasing glutamate excitotoxicity. We further studied the underlying mechanisms and effects of CTX early post-treatment on behavior in a cerebral hypoperfusion rats. The rats' common carotid arteries (2VO) were permanently ligated. CTX was treated after ischemia. Biochemical studies were performed to assess antioxidative stress and inflammation. Behavioral and histological studies were then tested on the ninth week after vessel ligation. The 2VO rats showed learning and memory deficits as well as working memory impairments without any motor weakness. The treatment with CTX was found to attenuate white matter damage, MDA production, and interleukin 1 beta and tumor necrosis factor alpha production, mainly in the hippocampal area. Moreover, CTX treatment could increase the expression of glia and the glial glutamate transporters, and the neuronal glutamate transporter. Taken together, our data indicate the neuroprotective mechanisms of CTX involving the upregulation of glutamate transporters' expression. This increased expression contributes to a reduction in glutamate excitotoxicity and oxidative stress as well as pro-inflammatory cytokine production, thus resulting in the protection of neurons and tissue from further damage. The present study highlights the mechanism of the effect of CTX treatment and of the underlying ischemia-induced neuronal damage.
Keywords: ceftriaxone; inflammation; oxidative stress; white matter damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Neuroprotective effect of ceftriaxone on the penumbra in a rat venous ischemia model.Neuroscience. 2013 Jul 9;242:1-10. doi: 10.1016/j.neuroscience.2013.03.018. Epub 2013 Mar 21. Neuroscience. 2013. PMID: 23523747
-
The beta-lactam antibiotic, ceftriaxone, provides neuroprotective potential via anti-excitotoxicity and anti-inflammation response in a rat model of traumatic brain injury.J Trauma Acute Care Surg. 2012 Sep;73(3):654-60. doi: 10.1097/TA.0b013e31825133c0. J Trauma Acute Care Surg. 2012. PMID: 22710775
-
Effects of melatonin on cognitive impairment and hippocampal neuronal damage in a rat model of chronic cerebral hypoperfusion.Exp Ther Med. 2016 Jun;11(6):2240-2246. doi: 10.3892/etm.2016.3216. Epub 2016 Apr 1. Exp Ther Med. 2016. PMID: 27284307 Free PMC article.
-
Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy.J Neural Transm (Vienna). 2014 Aug;121(8):1029-75. doi: 10.1007/s00702-014-1193-3. Epub 2014 Aug 1. J Neural Transm (Vienna). 2014. PMID: 25081016 Review.
-
Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases.Brain Res Rev. 2007 Apr;54(1):162-80. doi: 10.1016/j.brainresrev.2007.01.003. Epub 2007 Jan 18. Brain Res Rev. 2007. PMID: 17296232 Review.
Cited by
-
Crosstalk Between Autophagy and Inflammation in Chronic Cerebral Ischaemia.Cell Mol Neurobiol. 2023 Aug;43(6):2557-2566. doi: 10.1007/s10571-023-01336-6. Epub 2023 Mar 23. Cell Mol Neurobiol. 2023. PMID: 36952071 Free PMC article. Review.
-
Ceftriaxone has a neuroprotective effect in a whole-brain irradiation-induced neurotoxicity model by increasing GLT-1 and reducing oxidative stress.Strahlenther Onkol. 2025 Sep;201(9):903-919. doi: 10.1007/s00066-025-02405-z. Epub 2025 May 12. Strahlenther Onkol. 2025. PMID: 40353856
References
Grants and funding
LinkOut - more resources
Full Text Sources

