Laser Bioprinting of Cells Using UV and Visible Wavelengths: A Comparative DNA Damage Study
- PMID: 36004903
- PMCID: PMC9405344
- DOI: 10.3390/bioengineering9080378
Laser Bioprinting of Cells Using UV and Visible Wavelengths: A Comparative DNA Damage Study
Abstract
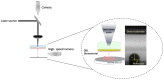
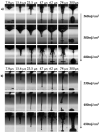
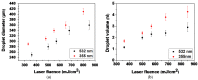
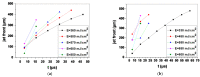
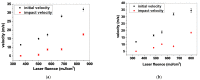
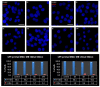
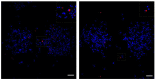
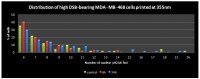
Laser-based techniques for printing cells onto different substrates with high precision and resolution present unique opportunities for contributing to a wide range of biomedical applications, including tissue engineering. In this study, laser-induced forward transfer (LIFT) printing was employed to rapidly and accurately deposit patterns of cancer cells in a non-contact manner, using two different wavelengths, 532 and 355 nm. To evaluate the effect of LIFT on the printed cells, their growth and DNA damage profiles were assessed and evaluated quantitatively over several days. The damaging effect of LIFT-printing was thoroughly investigated, for the first time at a single cell level, by counting individual double strand breaks (DSB). Overall, we found that LIFT was able to safely print patterns of breast cancer cells with high viability with little or no heat or shear damage to the cells, as indicated by unperturbed growth and negligible gross DNA damage.
Keywords: DNA damage; double strand breaks; laser fluence; laser-induced forward transfer (LIFT); wavelength.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Laser-induced forward transfer based laser bioprinting in biomedical applications.Front Bioeng Biotechnol. 2023 Aug 21;11:1255782. doi: 10.3389/fbioe.2023.1255782. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37671193 Free PMC article. Review.
-
Study of gelatin as an effective energy absorbing layer for laser bioprinting.Biofabrication. 2017 Jun 9;9(2):024103. doi: 10.1088/1758-5090/aa74f2. Biofabrication. 2017. PMID: 28597844
-
Parametric Study of Jet/Droplet Formation Process during LIFT Printing of Living Cell-Laden Bioink.Micromachines (Basel). 2021 Nov 16;12(11):1408. doi: 10.3390/mi12111408. Micromachines (Basel). 2021. PMID: 34832817 Free PMC article.
-
Laser printing of single cells: statistical analysis, cell viability, and stress.Ann Biomed Eng. 2005 Feb;33(2):121-30. doi: 10.1007/s10439-005-8971-x. Ann Biomed Eng. 2005. PMID: 15771266
-
Liquid-Phase Laser Induced Forward Transfer for Complex Organic Inks and Tissue Engineering.Ann Biomed Eng. 2017 Jan;45(1):84-99. doi: 10.1007/s10439-016-1617-3. Epub 2016 Apr 18. Ann Biomed Eng. 2017. PMID: 27090894 Review.
Cited by
-
Laser transfer for circulating tumor cell isolation in liquid biopsy.Int J Bioprint. 2023 Mar 28;9(4):720. doi: 10.18063/ijb.720. eCollection 2023. Int J Bioprint. 2023. PMID: 37323505 Free PMC article.
-
Advances in Organoid Research and Developmental Engineering.Bioengineering (Basel). 2024 Dec 15;11(12):1275. doi: 10.3390/bioengineering11121275. Bioengineering (Basel). 2024. PMID: 39768093 Free PMC article.
-
Laser Bioprinting with Cell Spheroids: Accurate and Gentle.Micromachines (Basel). 2023 May 30;14(6):1152. doi: 10.3390/mi14061152. Micromachines (Basel). 2023. PMID: 37374737 Free PMC article.
-
Three-dimensional bioprinting in ophthalmic care.Int J Ophthalmol. 2023 Oct 18;16(10):1702-1711. doi: 10.18240/ijo.2023.10.21. eCollection 2023. Int J Ophthalmol. 2023. PMID: 37854366 Free PMC article. Review.
-
Photocrosslinkable Biomaterials for 3D Bioprinting: Mechanisms, Recent Advances, and Future Prospects.Int J Mol Sci. 2024 Nov 22;25(23):12567. doi: 10.3390/ijms252312567. Int J Mol Sci. 2024. PMID: 39684279 Free PMC article. Review.
References
-
- Ringeisen B.R., Pirlo R.K., Wu P.K., Boland T., Huang Y., Sun W., Hamid Q., Chrisey D.B. Cell and organ printing turn s 15: Diverse research to commercial transitions. MRS Bull. 2013;38:834.
Grants and funding
LinkOut - more resources
Full Text Sources

