The Simultaneous Detection of Multiple Antibiotics in Milk and Pork Based on an Antibody Chip Biosensor
- PMID: 36004974
- PMCID: PMC9405744
- DOI: 10.3390/bios12080578
The Simultaneous Detection of Multiple Antibiotics in Milk and Pork Based on an Antibody Chip Biosensor
Abstract
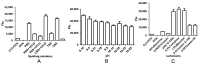
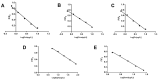
In the modern farming industry, the irrational or illegal use of veterinary drugs leads to residues in animal-derived food, which can seriously threaten human health. Efficient detection of low concentrations of drug residues in animal products in a short time is a key challenge for analytical methods. This study proposes to use an antibody chip biosensor for rapid and automated analysis of cephalosporins, aminoglycosides, and sulfonamide antibiotics in pork and milk. 3D polymer slides were applied for the preparation of antibody chips. Ovalbumin (OVA) or bovine serum albumin (BSA) conjugates of the haptens were immobilized as spots on disposable chips. Monoclonal antibodies (mAbs) against cefalexin, ceftiofur, gentamicin, neomycin, and sulfonamides allowed the simultaneous detection of the respective analytes. Antibody binding was detected by a second antibody labeled with Cy3-generating fluorescence, which was scanned a with chip scanner. The limits of detection (LOD) for all the analytes were far below the respective maximum residue limits (MRLs) and ranged from 0.51 to 4.3 µg/kg. The average recoveries of all the analytes in each sample were in the range of 81.6-113.6%. The intra- and inter-assay CV was less than 12.9% and showed good accuracy and precision for all the antibiotics at the MRL level. The sample pretreatment method is simple, and the results are confirmed to be accurate by LC-MS/MS; therefore, this method is valuable for the quality control of animal-derived food.
Keywords: aminoglycosides; antibody chip; cephalosporins; multi-residues; sulfonamides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Automated microarray system for the simultaneous detection of antibiotics in milk.Anal Chem. 2004 Feb 1;76(3):646-54. doi: 10.1021/ac035028i. Anal Chem. 2004. PMID: 14750859
-
Determination of aminoglycoside residues in milk and muscle based on a simple and fast extraction procedure followed by liquid chromatography coupled to tandem mass spectrometry and time of flight mass spectrometry.Talanta. 2016 Jul 1;154:38-45. doi: 10.1016/j.talanta.2016.03.045. Epub 2016 Mar 15. Talanta. 2016. PMID: 27154646
-
Simultaneous determination of clanobutin, dichlorvos, and naftazone in pork, beef, chicken, milk, and egg using liquid chromatography-tandem mass spectrometry.Food Chem. 2018 Jun 30;252:40-48. doi: 10.1016/j.foodchem.2018.01.085. Epub 2018 Jan 12. Food Chem. 2018. PMID: 29478562
-
Method validation, residue analysis and dietary risk assessment of trifloxystrobin and trifloxystrobin acid in milk, eggs and pork.Biomed Chromatogr. 2022 May;36(5):e5342. doi: 10.1002/bmc.5342. Epub 2022 Feb 4. Biomed Chromatogr. 2022. PMID: 35064586
-
Fully automated analysis of beta-lactams in bovine milk by online solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry.Anal Chem. 2009 Jun 1;81(11):4285-95. doi: 10.1021/ac9001386. Anal Chem. 2009. PMID: 19402673
Cited by
-
Simultaneous Screening of Six Families of Antibiotic Residues in Milk Samples by Biochip Multi-array Technology.Iran J Pharm Res. 2023 Aug 20;22(1):e136363. doi: 10.5812/ijpr-136363. eCollection 2023 Jan-Dec. Iran J Pharm Res. 2023. PMID: 38116570 Free PMC article.
-
A systematic review of sensors to combat crime and routes to further sensor development.Front Chem. 2025 Jun 12;13:1568867. doi: 10.3389/fchem.2025.1568867. eCollection 2025. Front Chem. 2025. PMID: 40575464 Free PMC article. Review.
References
-
- Hayer S.S., Casanova-Higes A., Paladino E., Elnekave E., Nault A., Johnson T., Bender J., Perez A., Alvarez J. Global Distribution of Extended Spectrum Cephalosporin and Carbapenem Resistance and Associated Resistance Markers in Escherichia coli of Swine Origin—A Systematic Review and Meta-Analysis. Front. Microbiol. 2022;13:1684. doi: 10.3389/fmicb.2022.853810. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

