Miniaturized Raman Instruments for SERS-Based Point-of-Care Testing on Respiratory Viruses
- PMID: 36004986
- PMCID: PMC9405795
- DOI: 10.3390/bios12080590
Miniaturized Raman Instruments for SERS-Based Point-of-Care Testing on Respiratory Viruses
Abstract
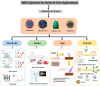
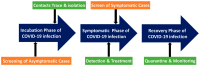
As surface-enhanced Raman scattering (SERS) has been used to diagnose several respiratory viruses (e.g., influenza A virus subtypes such as H1N1 and the new coronavirus SARS-CoV-2), SERS is gaining popularity as a method for diagnosing viruses at the point-of-care. Although the prior and quick diagnosis of respiratory viruses is critical in the outbreak of infectious disease, ELISA, PCR, and RT-PCR have been used to detect respiratory viruses for pandemic control that are limited for point-of-care testing. SERS provides quantitative data with high specificity and sensitivity in a real-time, label-free, and multiplex manner recognizing molecular fingerprints. Recently, the design of Raman spectroscopy system was simplified from a complicated design to a small and easily accessible form that enables point-of-care testing. We review the optical design (e.g., laser wavelength/power and detectors) of commercialized and customized handheld Raman instruments. As respiratory viruses have prominent risk on the pandemic, we review the applications of handheld Raman devices for detecting respiratory viruses. By instrumentation and commercialization advancements, the advent of the portable SERS device creates a fast, accurate, practical, and cost-effective analytical method for virus detection, and would continue to attract more attention in point-of-care testing.
Keywords: Raman scattering; pandemic; point-of-care testing device; respiratory viruses; surface-enhanced Raman spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

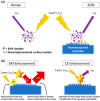
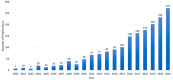
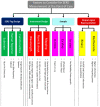
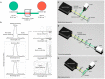





References
-
- Singh R. CV raman and the discovery of the raman effect. Phys. Perspect. 2002;4:399–420. doi: 10.1007/s000160200002. - DOI
-
- Long D.A. Early history of the raman effect. Int. Rev. Phys. Chem. 1988;7:317–349. doi: 10.1080/01442358809353216. - DOI
-
- Adar F., Delhaye M., DaSilva E. Evolution of instrumentation for detection of the raman effect as driven by available technologies and by developing applications. J. Chem. Educ. 2007;84:50. doi: 10.1021/ed084p50. - DOI
-
- Sweedler J.V. Charge transfer device detectors and their applications to chemical analysis. Crit. Rev. Anal. Chem. 1993;24:59–98. doi: 10.1080/10408349308048819. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

