Determination of rSpike Protein by Specific Antibodies with Screen-Printed Carbon Electrode Modified by Electrodeposited Gold Nanostructures
- PMID: 36004989
- PMCID: PMC9405582
- DOI: 10.3390/bios12080593
Determination of rSpike Protein by Specific Antibodies with Screen-Printed Carbon Electrode Modified by Electrodeposited Gold Nanostructures
Abstract
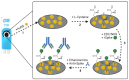
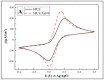
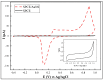
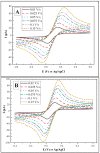
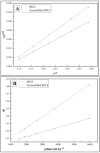
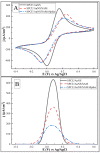
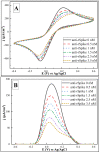
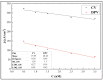
In this research, we assessed the applicability of electrochemical sensing techniques for detecting specific antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike proteins in the blood serum of patient samples following coronavirus disease 2019 (COVID-19). Herein, screen-printed carbon electrodes (SPCE) with electrodeposited gold nanostructures (AuNS) were modified with L-Cysteine for further covalent immobilization of recombinant SARS-CoV-2 spike proteins (rSpike). The affinity interactions of the rSpike protein with specific antibodies against this protein (anti-rSpike) were assessed using cyclic voltammetry (CV) and differential pulse voltammetry (DPV) methods. It was revealed that the SPCE electroactive surface area increased from 1.49 ± 0.02 cm2 to 1.82 ± 0.01 cm2 when AuNS were electrodeposited, and the value of the heterogeneous electron transfer rate constant (k0) changed from 6.30 × 10-5 to 14.56 × 10-5. The performance of the developed electrochemical immunosensor was evaluated by calculating the limit of detection and limit of quantification, giving values of 0.27 nM and 0.81 nM for CV and 0.14 nM and 0.42 nM for DPV. Furthermore, a specificity test was performed with a solution of antibodies against bovine serum albumin as the control aliquot, which was used to assess nonspecific binding, and this evaluation revealed that the developed rSpike-based sensor exhibits low nonspecific binding towards anti-rSpike antibodies.
Keywords: COVID-19; SARS-CoV-2 virus; antigen–antibody complex; cyclic voltammetry (CV); differential pulse voltammetry (DPV); electrochemical immunosensor; electrochemical impedance spectroscopy (EIS); gold nanostructures (AuNS); self-assembled monolayer (SAM); spike protein (Spike).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Yadav A., Verma D., Kumar A., Kumar P., Solanki P. The perspectives of biomarker-based electrochemical immunosensors, artificial intelligence and the Internet of Medical Things toward COVID-19 diagnosis and management. Mater. Today Chem. 2021;20:100443. doi: 10.1016/j.mtchem.2021.100443. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

