Measurements of Anti-SARS-CoV-2 Antibody Levels after Vaccination Using a SH-SAW Biosensor
- PMID: 36004995
- PMCID: PMC9405803
- DOI: 10.3390/bios12080599
Measurements of Anti-SARS-CoV-2 Antibody Levels after Vaccination Using a SH-SAW Biosensor
Abstract
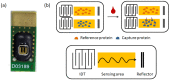
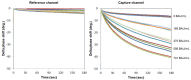
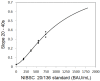
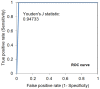
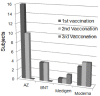
To prevent the COVID-19 pandemic that threatens human health, vaccination has become a useful and necessary tool in the response to the pandemic. The vaccine not only induces antibodies in the body, but may also cause adverse effects such as fatigue, muscle pain, blood clots, and myocarditis, especially in patients with chronic disease. To reduce unnecessary vaccinations, it is becoming increasingly important to monitor the amount of anti-SARS-CoV-2 S protein antibodies prior to vaccination. A novel SH-SAW biosensor, coated with SARS-CoV-2 spike protein, can help quantify the amount of anti-SARS-CoV-2 S protein antibodies with 5 μL of finger blood within 40 s. The LoD of the spike-protein-coated SAW biosensor was determined to be 41.91 BAU/mL, and the cut-off point was determined to be 50 BAU/mL (Youden’s J statistic = 0.94733). By using the SH-SAW biosensor, we found that the total anti-SARS-CoV-2 S protein antibody concentrations spiked 10−14 days after the first vaccination (p = 0.0002) and 7−9 days after the second vaccination (p = 0.0116). Furthermore, mRNA vaccines, such as Moderna or BNT, could achieve higher concentrations of total anti-SARS-CoV-2 S protein antibodies compared with adenovirus vaccine, AZ (p < 0.0001). SH-SAW sensors in vitro diagnostic systems are a simple and powerful technology to investigate the local prevalence of COVID-19.
Keywords: SARS-CoV-2; SH-SAW biosensor; antibody; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
[Antibody Response After Two Doses of Inactivated SARS-CoV-2 Vaccine in Healthcare Workers with and without Previous COVID-19 Infection: A Prospective Observational Study].Mikrobiyol Bul. 2022 Jan;56(1):36-48. doi: 10.5578/mb.20229904. Mikrobiyol Bul. 2022. PMID: 35088958 Turkish.
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Long-Term Humoral Immune Response against SARS-CoV-2 after Natural Infection and Subsequent Vaccination According to WHO International Binding Antibody Units (BAU/mL).Viruses. 2021 Nov 23;13(12):2336. doi: 10.3390/v13122336. Viruses. 2021. PMID: 34960605 Free PMC article.
-
Anti-SARS-CoV-2 spike IgG following injection of the third dose vaccine: A systematic review with meta-analysis of heterologous versus homologous vaccination.Front Public Health. 2023 Jan 12;10:960598. doi: 10.3389/fpubh.2022.960598. eCollection 2022. Front Public Health. 2023. PMID: 36711369 Free PMC article.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
Cited by
-
Application of Shear Horizontal Surface Acoustic Wave (SH-SAW) Immunosensor in Point-of-Care Diagnosis.Biosensors (Basel). 2023 Jun 1;13(6):605. doi: 10.3390/bios13060605. Biosensors (Basel). 2023. PMID: 37366970 Free PMC article. Review.
-
New Advances in Rapid Pretreatment for Small Dense LDL Cholesterol Measurement Using Shear Horizontal Surface Acoustic Wave (SH-SAW) Technology.Int J Mol Sci. 2024 Jan 15;25(2):1044. doi: 10.3390/ijms25021044. Int J Mol Sci. 2024. PMID: 38256117 Free PMC article.
-
Fundamentals of SARS-CoV-2 Biosensors.Biosensors (Basel). 2022 Oct 17;12(10):880. doi: 10.3390/bios12100880. Biosensors (Basel). 2022. PMID: 36291016 Free PMC article.
-
Tracking the Risk of Cardiovascular Disease after Almond and Oat Milk Intervene or Statin Medication with a Powerful Reflex SH-SAW POCT Platform.Sensors (Basel). 2024 Oct 10;24(20):6517. doi: 10.3390/s24206517. Sensors (Basel). 2024. PMID: 39459999 Free PMC article.
-
ZnO-Based Electrochemical Immunosensor to Assess Vaccine-Induced Antibody-Mediated Immunity against Wild-Type and Gamma SARS-CoV-2 Strains.Biosensors (Basel). 2023 Mar 11;13(3):371. doi: 10.3390/bios13030371. Biosensors (Basel). 2023. PMID: 36979583 Free PMC article.
References
-
- Van Elslande J., Decru B., Jonckheere S., Van Wijngaerden E., Houben E., Vandecandelaere P., Indevuyst C., Depypere M., Desmet S., Andre E., et al. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin. Microbiol. Infect. 2020;26:1557.e1–1557.e7. doi: 10.1016/j.cmi.2020.07.038. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

