Measurements of Anti-SARS-CoV-2 Antibody Levels after Vaccination Using a SH-SAW Biosensor
- PMID: 36004995
- PMCID: PMC9405803
- DOI: 10.3390/bios12080599
Measurements of Anti-SARS-CoV-2 Antibody Levels after Vaccination Using a SH-SAW Biosensor
Abstract
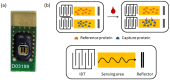
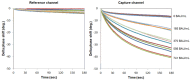
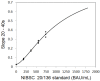
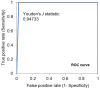
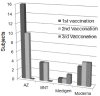
To prevent the COVID-19 pandemic that threatens human health, vaccination has become a useful and necessary tool in the response to the pandemic. The vaccine not only induces antibodies in the body, but may also cause adverse effects such as fatigue, muscle pain, blood clots, and myocarditis, especially in patients with chronic disease. To reduce unnecessary vaccinations, it is becoming increasingly important to monitor the amount of anti-SARS-CoV-2 S protein antibodies prior to vaccination. A novel SH-SAW biosensor, coated with SARS-CoV-2 spike protein, can help quantify the amount of anti-SARS-CoV-2 S protein antibodies with 5 μL of finger blood within 40 s. The LoD of the spike-protein-coated SAW biosensor was determined to be 41.91 BAU/mL, and the cut-off point was determined to be 50 BAU/mL (Youden’s J statistic = 0.94733). By using the SH-SAW biosensor, we found that the total anti-SARS-CoV-2 S protein antibody concentrations spiked 10−14 days after the first vaccination (p = 0.0002) and 7−9 days after the second vaccination (p = 0.0116). Furthermore, mRNA vaccines, such as Moderna or BNT, could achieve higher concentrations of total anti-SARS-CoV-2 S protein antibodies compared with adenovirus vaccine, AZ (p < 0.0001). SH-SAW sensors in vitro diagnostic systems are a simple and powerful technology to investigate the local prevalence of COVID-19.
Keywords: SARS-CoV-2; SH-SAW biosensor; antibody; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Van Elslande J., Decru B., Jonckheere S., Van Wijngaerden E., Houben E., Vandecandelaere P., Indevuyst C., Depypere M., Desmet S., Andre E., et al. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin. Microbiol. Infect. 2020;26:1557.e1–1557.e7. doi: 10.1016/j.cmi.2020.07.038. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

