Electrochemical Biosensor for SARS-CoV-2 cDNA Detection Using AuPs-Modified 3D-Printed Graphene Electrodes
- PMID: 36005018
- PMCID: PMC9405530
- DOI: 10.3390/bios12080622
Electrochemical Biosensor for SARS-CoV-2 cDNA Detection Using AuPs-Modified 3D-Printed Graphene Electrodes
Abstract
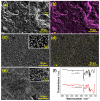
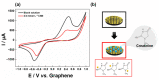
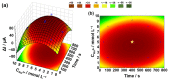
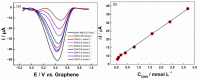
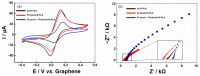
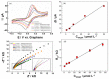
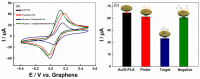
A low-cost and disposable graphene polylactic (G-PLA) 3D-printed electrode modified with gold particles (AuPs) was explored to detect the cDNA of SARS-CoV-2 and creatinine, a potential biomarker for COVID-19. For that, a simple, non-enzymatic electrochemical sensor, based on a Au-modified G-PLA platform was applied. The AuPs deposited on the electrode were involved in a complexation reaction with creatinine, resulting in a decrease in the analytical response, and thus providing a fast and simple electroanalytical device. Physicochemical characterizations were performed by SEM, EIS, FTIR, and cyclic voltammetry. Square wave voltammetry was employed for the creatinine detection, and the sensor presented a linear response with a detection limit of 0.016 mmol L-1. Finally, a biosensor for the detection of SARS-CoV-2 was developed based on the immobilization of a capture sequence of the viral cDNA upon the Au-modified 3D-printed electrode. The concentration, immobilization time, and hybridization time were evaluated in presence of the DNA target, resulting in a biosensor with rapid and low-cost analysis, capable of sensing the cDNA of the virus with a good limit of detection (0.30 µmol L-1), and high sensitivity (0.583 µA µmol-1 L). Reproducible results were obtained (RSD = 1.14%, n = 3), attesting to the potentiality of 3D-printed platforms for the production of biosensors.
Keywords: 3D printed electrode; AuP modified electrode; SARS-CoV-2; creatinine; electrochemical (bio)sensor.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures

Similar articles
-
3D-printed reduced graphene oxide/polylactic acid electrodes: A new prototyped platform for sensing and biosensing applications.Biosens Bioelectron. 2020 Dec 15;170:112684. doi: 10.1016/j.bios.2020.112684. Epub 2020 Oct 8. Biosens Bioelectron. 2020. PMID: 33049481
-
Voltammetric-based immunosensor for the detection of SARS-CoV-2 nucleocapsid antigen.Mikrochim Acta. 2021 May 26;188(6):199. doi: 10.1007/s00604-021-04867-1. Mikrochim Acta. 2021. PMID: 34041585 Free PMC article.
-
A label-free electrochemical DNA biosensor used a printed circuit board gold electrode (PCBGE) to detect SARS-CoV-2 without amplification.Lab Chip. 2023 Mar 14;23(6):1622-1636. doi: 10.1039/d2lc01159j. Lab Chip. 2023. PMID: 36786757
-
A Short Review Comparing Carbon-Based Electrochemical Platforms With Other Materials For Biosensing SARS-Cov-2.ChemistrySelect. 2022 Oct 7;7(37):e202202465. doi: 10.1002/slct.202202465. Epub 2022 Oct 4. ChemistrySelect. 2022. PMID: 36711230 Free PMC article. Review.
-
New approach in SARS-CoV-2 surveillance using biosensor technology: a review.Environ Sci Pollut Res Int. 2022 Jan;29(2):1677-1695. doi: 10.1007/s11356-021-17096-z. Epub 2021 Oct 23. Environ Sci Pollut Res Int. 2022. PMID: 34689274 Free PMC article. Review.
Cited by
-
3D-printed electrochemical glucose device with integrated Fe(II)-MOF nanozyme.Mikrochim Acta. 2023 Jun 24;190(7):274. doi: 10.1007/s00604-023-05860-6. Mikrochim Acta. 2023. PMID: 37354230 Free PMC article.
-
Electrochemical Biosensors 3D Printed by Fused Deposition Modeling: Actualities, Trends, and Challenges.Biosensors (Basel). 2025 Jan 17;15(1):57. doi: 10.3390/bios15010057. Biosensors (Basel). 2025. PMID: 39852108 Free PMC article. Review.
-
Electrochemical Sensor Based on Laser-Induced Graphene for Carbendazim Detection in Water.Foods. 2023 Jun 6;12(12):2277. doi: 10.3390/foods12122277. Foods. 2023. PMID: 37372489 Free PMC article.
-
Emerging Biomedical and Clinical Applications of 3D-Printed Poly(Lactic Acid)-Based Devices and Delivery Systems.Bioengineering (Basel). 2024 Jul 11;11(7):705. doi: 10.3390/bioengineering11070705. Bioengineering (Basel). 2024. PMID: 39061787 Free PMC article. Review.
-
Parkinson biomarker determination with an Au microflower-enhanced electrochemical immunosensor using non-Faradaic capacitance measurements.Mikrochim Acta. 2024 Oct 11;191(11):663. doi: 10.1007/s00604-024-06747-w. Mikrochim Acta. 2024. PMID: 39392501
References
-
- Stefano J.S., Kalinke C., da Rocha R.G., Rocha D.P., da Silva V.A.O.P., Bonacin J.A., Angnes L., Richter E.M., Janegitz B.C., Muñoz R.A.A. Electrochemical (bio)sensors enabled by fused deposition modeling-based 3D printing: A guide to selecting designs, printing parameters, and post-treatment protocols. Anal. Chem. 2022;94:6417–6429. doi: 10.1021/acs.analchem.1c05523. - DOI - PubMed
-
- Liyarita B.R., Ambrosi A., Pumera M. 3D-printed electrodes for sensing of biologically active molecules. Wiley Online Libr. 2018;30:1319–1326. doi: 10.1002/elan.201700828. - DOI
-
- Whittingham M.J., Crapnell R.D., Rothwell E.J., Hurst N.J., Banks C.E. Additive manufacturing for electrochemical labs: An overview and tutorial note on the production of cells, electrodes and accessories. Talanta Open. 2021;4:100051. doi: 10.1016/j.talo.2021.100051. - DOI
-
- Jayaprakash G.K. Pre-post redox electron transfer regioselectivity at the alanine modified nano graphene electrode interface. Chem. Phys. Lett. 2022;789:139295. doi: 10.1016/j.cplett.2021.139295. - DOI
MeSH terms
Substances
Grants and funding
- 2017/21097-3/São Paulo Research Foundation
- 2022/06145-0/São Paulo Research Foundation
- 2018/19750-3/São Paulo Research Foundation
- 001/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- 88887.510506/2020-00/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- 88887.510880/2020-00/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- 303338/2019-9/National Council for Scientific and Technological Development
- 427731/2018-6/National Council for Scientific and Technological Development
- 307271/2017-0/National Council for Scientific and Technological Development
- 465389/2014-7/National Council for Scientific and Technological Development
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

