Fluorescence-Based Microendoscopic Sensing System for Minimally Invasive In Vivo Bladder Cancer Diagnosis
- PMID: 36005027
- PMCID: PMC9406178
- DOI: 10.3390/bios12080631
Fluorescence-Based Microendoscopic Sensing System for Minimally Invasive In Vivo Bladder Cancer Diagnosis
Abstract
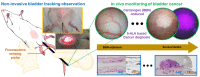
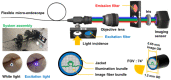
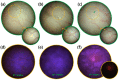
Bladder cancer is commonly diagnosed by evaluating the tissue morphology through cystoscopy, and tumor resection is used as the primary treatment approach. However, these methods are limited by lesion site specificity and resection margin, and can thereby fail to detect cancer lesions at early stages. Nevertheless, rapid diagnosis without biopsy may be possible through fluorescence sensing. Herein, we describe a minimally invasive imaging system capable of sensing even small tumors through a 1.2 mm diameter flexible fiber bundle microprobe. We demonstrate that this new device can be used for the early diagnosis of bladder cancer in rats. Bladder cancer was induced in rats using the carcinogen N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN), and a togglable filter capable of PpIX fluorescence sensing was installed in the microendoscopic system. Following 5-aminolevulinic acid administration, tissue in the early stages of bladder cancer was successfully identified with fluorescence detection and confirmed with hematoxylin/eosin and ferrochelatase staining. Although the time required for BBN to induce bladder cancer varied between 3 and 4 weeks among the rats, the microendoscopic system allowed the minimally invasive follow-up on cancer development.
Keywords: 5-aminolevulinic acid; bladder cancer; ferrochelatase staining; microendoscopy; minimally invasive diagnosis; protoporphyrin IX.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Expression of ferrochelatase has a strong correlation in protoporphyrin IX accumulation with photodynamic detection of bladder cancer.Photodiagnosis Photodyn Ther. 2016 Mar;13:225-232. doi: 10.1016/j.pdpdt.2015.07.174. Epub 2015 Jul 28. Photodiagnosis Photodyn Ther. 2016. PMID: 26226642
-
Fluorescence cystoscopy following intravesical instillation of 5-aminolevulinic acid: a new procedure with high sensitivity for detection of hardly visible urothelial neoplasias.Urol Int. 1995;55(4):190-6. doi: 10.1159/000282784. Urol Int. 1995. PMID: 8588264
-
Fluorescence diagnosis of bladder cancer: a novel in vivo approach using 5-aminolevulinic acid (ALA) dendrimers.BJU Int. 2012 Dec;110(11 Pt C):E1155-62. doi: 10.1111/j.1464-410X.2012.11407.x. Epub 2012 Aug 10. BJU Int. 2012. PMID: 22883132
-
New optical imaging technologies for bladder cancer: considerations and perspectives.J Urol. 2012 Aug;188(2):361-8. doi: 10.1016/j.juro.2012.03.127. Epub 2012 Jun 13. J Urol. 2012. PMID: 22698620 Free PMC article. Review.
-
Endoscopic fluorescence diagnosis and laser treatment of transitional cell carcinoma of the bladder.Semin Urol Oncol. 2000 Nov;18(4):264-72. Semin Urol Oncol. 2000. PMID: 11101089 Review.
Cited by
-
Biophotonics as a new application in optical technology: A bibliometric analysis.Heliyon. 2023 Nov 29;9(12):e23011. doi: 10.1016/j.heliyon.2023.e23011. eCollection 2023 Dec. Heliyon. 2023. PMID: 38076099 Free PMC article. Review.
-
Nanotechnology and Cancer Bioelectricity: Bridging the Gap Between Biology and Translational Medicine.Adv Sci (Weinh). 2024 Jan;11(1):e2304110. doi: 10.1002/advs.202304110. Epub 2023 Nov 20. Adv Sci (Weinh). 2024. PMID: 37984883 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

