Recent Advances in Fluorescent Methods for Polyamine Detection and the Polyamine Suppressing Strategy in Tumor Treatment
- PMID: 36005029
- PMCID: PMC9405807
- DOI: 10.3390/bios12080633
Recent Advances in Fluorescent Methods for Polyamine Detection and the Polyamine Suppressing Strategy in Tumor Treatment
Abstract
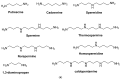
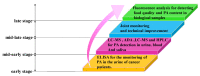
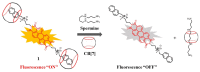
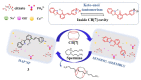
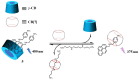
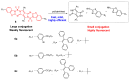
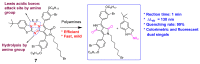
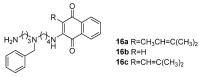
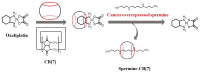
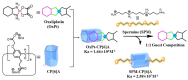
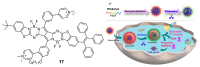
The biogenic aliphatic polyamines (spermine, spermidine, and putrescine) are responsible for numerous cell functions, including cell proliferation, the stabilization of nucleic acid conformations, cell division, homeostasis, gene expression, and protein synthesis in living organisms. The change of polyamine concentrations in the urine or blood is usually related to the presence of malignant tumors and is regarded as a biomarker for the early diagnosis of cancer. Therefore, the detection of polyamine levels in physiological fluids can provide valuable information in terms of cancer diagnosis and in monitoring therapeutic effects. In this review, we summarize the recent advances in fluorescent methods for polyamine detection (supramolecular fluorescent sensing systems, fluorescent probes based on the chromophore reaction, fluorescent small molecules, and fluorescent nanoparticles). In addition, tumor polyamine-suppressing strategies (such as polyamine conjugate, polyamine analogs, combinations that target multiple components, spermine-responsive supramolecular chemotherapy, a combination of polyamine consumption and photodynamic therapy, etc.) are highlighted. We hope that this review promotes the development of more efficient polyamine detection methods and provides a comprehensive understanding of polyamine-based tumor suppressor strategies.
Keywords: detection; polyamines; suppressor strategies; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

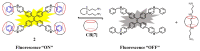

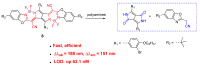
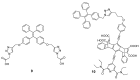
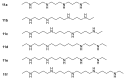

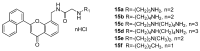
References
-
- Jastrzab R., Kaczmarek M.T., Nowak M., Trojanowska A., Zabiszak M. Complexes of Polyamines and Their Derivatives as Living System Active Compounds. Coord. Chem. Rev. 2017;351:32–44. doi: 10.1016/j.ccr.2017.05.001. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

