Liquid Crystal Biosensors: Principles, Structure and Applications
- PMID: 36005035
- PMCID: PMC9406233
- DOI: 10.3390/bios12080639
Liquid Crystal Biosensors: Principles, Structure and Applications
Abstract
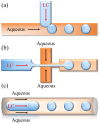
Liquid crystals (LCs) have been widely used as sensitive elements to construct LC biosensors based on the principle that specific bonding events between biomolecules can affect the orientation of LC molecules. On the basis of the sensing interface of LC molecules, LC biosensors can be classified into three types: LC-solid interface sensing platforms, LC-aqueous interface sensing platforms, and LC-droplet interface sensing platforms. In addition, as a signal amplification method, the combination of LCs and whispering gallery mode (WGM) optical microcavities can provide higher detection sensitivity due to the extremely high quality factor and the small mode volume of the WGM optical microcavity, which enhances the interaction between the light field and biotargets. In this review, we present an overview of the basic principles, the structure, and the applications of LC biosensors. We discuss the important properties of LC and the principle of LC biosensors. The different geometries of LCs in the biosensing systems as well as their applications in the biological detection are then described. The fabrication and the application of the LC-based WGM microcavity optofluidic sensor in the biological detection are also introduced. Finally, challenges and potential research opportunities in the development of LC-based biosensors are discussed.
Keywords: LC-based biosensors; liquid crystals; microfluidics; optofluidic; whispering gallery mode.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Prévôt M.E., Nemati A., Cull T.R., Hegmann E., Hegmann T. A zero-power optical, ppt-to ppm-level toxic gas and vapor sensor with image, text, and analytical capabilities. Adv. Mater. Technol. 2020;5:2000058. doi: 10.1002/admt.202000058. - DOI
-
- Tefelska M.M., Woliński T.R., Ertman S., Mileńko K., ączkowski R., Siarkowska A., Domański A.W. Electric field sensing with photonic liquid crystal fibers based on micro-electrodes systems. J. Light. Technol. 2015;33:2405–2411. doi: 10.1109/JLT.2014.2379691. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

