Reactive Species-Activatable AIEgens for Biomedical Applications
- PMID: 36005044
- PMCID: PMC9406055
- DOI: 10.3390/bios12080646
Reactive Species-Activatable AIEgens for Biomedical Applications
Abstract
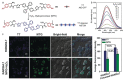
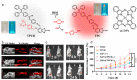
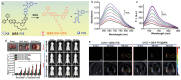
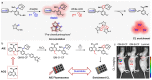
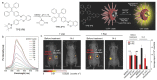
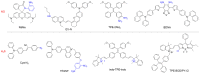
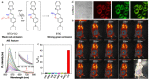
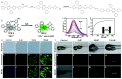
Precision medicine requires highly sensitive and specific diagnostic strategies with high spatiotemporal resolution. Accurate detection and monitoring of endogenously generated biomarkers at the very early disease stage is of extensive importance for precise diagnosis and treatment. Aggregation-induced emission luminogens (AIEgens) have emerged as a new type of excellent optical agents, which show great promise for numerous biomedical applications. In this review, we highlight the recent advances of AIE-based probes for detecting reactive species (including reactive oxygen species (ROS), reactive nitrogen species (RNS), reactive sulfur species (RSS), and reactive carbonyl species (RCS)) and related biomedical applications. The molecular design strategies for increasing the sensitivity, tuning the response wavelength, and realizing afterglow imaging are summarized, and theranostic applications in reactive species-related major diseases such as cancer, inflammation, and vascular diseases are reviewed. The challenges and outlooks for the reactive species-activatable AIE systems for disease diagnostics and therapeutics are also discussed. This review aims to offer guidance for designing AIE-based specifically activatable optical agents for biomedical applications, as well as providing a comprehensive understanding about the structure-property application relationships. We hope it will inspire more interesting researches about reactive species-activatable probes and advance clinical translations.
Keywords: activatable probe; afterglow; aggregation-induced emission; bioimaging; fluorescence; photoacoustic; reactive oxygen nitrogen species; theranostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

