A Preliminary Study about the Role of Reactive Oxygen Species and Inflammatory Process after COVID-19 Vaccination and COVID-19 Disease
- PMID: 36005066
- PMCID: PMC9406688
- DOI: 10.3390/clinpract12040063
A Preliminary Study about the Role of Reactive Oxygen Species and Inflammatory Process after COVID-19 Vaccination and COVID-19 Disease
Abstract
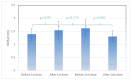
During the last couple of critical years, worldwide, there have been more than 550 million confirmed cases of COVID-19, including more than 6 million deaths (reported by the WHO); with respect to these cases, several vaccines, mainly mRNA vaccines, seem to prevent and protect from SARS-CoV-2 infection. We hypothesize that oxidative stress is one of the key factors playing an important role in both the generation and development of various kinds of disease, as well as antibody generation, as many biological paths can generate reactive oxygen species (ROS), and cellular activities can be modulated when ROS/antioxidant balance is interrupted. A pilot study was conducted in two stages during the COVID-19 pandemic in 2021 involving 222 participants between the ages of 26 and 66 years. ROS levels were measured before an after vaccination in the blood samples of 20 individuals who were vaccinated with two doses of mRNA vaccine, and an increase in ROS levels was observed after the first dose, with no modifications observed until the day before the second vaccination dose. A statistically significant difference (p < 0.001) was observed between time points 3 and 4 (before and after second dose), when participants were vaccinated for the second time, and ROS levels decreased from 21,758 to 17,580 a.u. In the second stage, blood was collected from 28 participants 45 days after COVID-19 infection (Group A), from 131 participants 45 days after receiving two doses of mRNA vaccine against COVID-19 (Group B), and from 13 healthy individuals as a control group (Group C). Additionally, antibodies levels were measured in all groups to investigate a possible correlation with ROS levels. A strong negative correlation was found between free radicals and disease antibodies in Group A (r = −0.45, p = 0.001), especially in the male subgroup (r = −0.88, p = 0.001), as well as in the female subgroup (r = −0.24, p < 0.001). Furthermore, no significant correlation (only a negative trend) was found with antibodies derived from vaccination in Group B (r = −0.01), and a negative trend was observed in the female subgroup, whereas a positive trend was observed in the male subgroup.
Keywords: COVID-19; ROS; antibodies; mRNA vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers.Lancet Respir Med. 2021 Sep;9(9):999-1009. doi: 10.1016/S2213-2600(21)00220-4. Epub 2021 Jul 2. Lancet Respir Med. 2021. PMID: 34224675 Free PMC article.
-
Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomised, double-blind, placebo-controlled, phase 1 trial.Lancet Microbe. 2022 Mar;3(3):e193-e202. doi: 10.1016/S2666-5247(21)00280-9. Epub 2022 Jan 24. Lancet Microbe. 2022. PMID: 35098177 Free PMC article. Clinical Trial.
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Are SARS-CoV-2 Antibodies Detectable in Human Milk After Vaccination Against COVID-19?J Pediatric Infect Dis Soc. 2022 Apr 30;11(4):126. doi: 10.1093/jpids/piac024. J Pediatric Infect Dis Soc. 2022. PMID: 35394545 Free PMC article.
Cited by
-
The Impact of Serum Levels of Reactive Oxygen and Nitrogen Species on the Disease Severity of COVID-19.Int J Mol Sci. 2023 May 18;24(10):8973. doi: 10.3390/ijms24108973. Int J Mol Sci. 2023. PMID: 37240319 Free PMC article.
-
Alternations in miR-155 and miR-200 serum levels can serve as biomarkers for COVID-19 in the post-mass vaccination era.Mol Biol Rep. 2024 May 25;51(1):689. doi: 10.1007/s11033-024-09630-2. Mol Biol Rep. 2024. PMID: 38796651
-
Catalase Activity of IgGs of Patients Infected with SARS-CoV-2.Int J Mol Sci. 2023 Jun 13;24(12):10081. doi: 10.3390/ijms241210081. Int J Mol Sci. 2023. PMID: 37373231 Free PMC article.
-
Assessment of Placental Antioxidant Defense Markers in Vaccinated and Unvaccinated COVID-19 Third-Trimester Pregnancies.Life (Basel). 2024 Nov 29;14(12):1571. doi: 10.3390/life14121571. Life (Basel). 2024. PMID: 39768279 Free PMC article.
References
-
- Tsang H.F., Chan L.W.C., Cho W.C.S., Yu A.C.S., Yim A.K.Y., Chan A.K.C., Ng L.P.W., Wong Y.K.E., Pei X.M., Li M.J.W., et al. An update on COVID-19 pandemic: The epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev. Anti-Infect. Ther. 2020;19:877–888. doi: 10.1080/14787210.2021.1863146. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous