Enhancing Mechanical Performance of a Polymer Material by Incorporating Pillar[5]arene-Based Host-Guest Interactions
- PMID: 36005076
- PMCID: PMC9407059
- DOI: 10.3390/gels8080475
Enhancing Mechanical Performance of a Polymer Material by Incorporating Pillar[5]arene-Based Host-Guest Interactions
Abstract
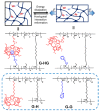
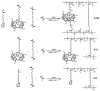
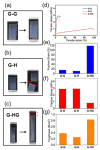
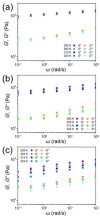
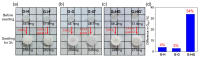
Polymer gels have been widely used in the field for tissue engineering, sensing, and drug delivery due to their excellent biocompatibility, hydrophilicity, and degradability. However, common polymer gels are easily deformed on account of their relatively weak mechanical properties, thereby hindering their application fields, as well as shortening their service life. The incorporation of reversible non-covalent bonds is capable of improving the mechanical properties of polymer gels. Thus, here, a poly(methyl methacrylate) polymer network was prepared by introducing host-guest interactions between pillar[5]arene and pyridine cation. Owing to the incorporated host-guest interactions, the modified polymer gels exhibited extraordinary mechanical properties according to the results of the tensile tests. In addition, the influence of the host-guest interaction on the mechanical properties of the gels was also proved by rheological experiments and swelling experiments.
Keywords: host–guest interactions; mechanical performance; pillar[5]arene; polymer gels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ji X., Jie K., Zimmerman S.C., Huang F. A double supramolecular crosslinked polymer gel exhibiting macroscale expansion and contraction behavior and multistimuli responsiveness. Polym. Chem. 2015;6:1912–1917. doi: 10.1039/C4PY01715C. - DOI
Grants and funding
- No. 22001087/National Natural Science Foundation of China
- grant 2020kfyXJJS013/Fundamental Research Funds for the Central Universities
- 2020MCF08/Open Fund of Hubei Key Laboratory of Material Chemistry and Service Failure, Huazhong University of Science and Technology
- No. 2021JYBKF01/Open Research Fund of Key Laboratory of Material Chemistry for Energy Conversion and Storage, Huazhong University of Science and Technology
LinkOut - more resources
Full Text Sources

