Fabrication of 3D Gelatin Hydrogel Nanocomposite Impregnated Co-Doped SnO2 Nanomaterial for the Catalytic Reduction of Environmental Pollutants
- PMID: 36005080
- PMCID: PMC9407077
- DOI: 10.3390/gels8080479
Fabrication of 3D Gelatin Hydrogel Nanocomposite Impregnated Co-Doped SnO2 Nanomaterial for the Catalytic Reduction of Environmental Pollutants
Abstract
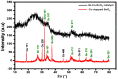
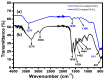
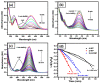
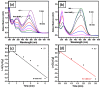
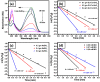
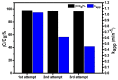
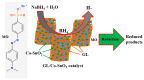
In the catalytic reduction of various environment pollutants, cobalt-doped tin oxide, i.e., Co-SnO2 intercalated gelatin (GL) hydrogel nanocomposite was prepared via direct mixing of Co-SnO2 doped with GL. Then, it was crosslinked internally using formaldehyde within a viscous solution of gelatin polymer, which led to the formation of GL/Co-SnO2 hydrogel nanocomposite. GL/Co-SnO2 hydrogel nanocomposite was fully characterized by using field-emission scanning electron microscopy (FESEM), energy dispersive X-ray spectroscopy (EDX), powder X-ray diffraction (XRD), and attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR). The FESEM images indicate that the Co-SnO2 composite has a spherical structure on the GL matrix while EDX elucidates the elemental composition of each atom in the crosslinked GL/Co-SnO2 hydrogel nanocomposite. The GL/Co-SnO2 nanocomposite was checked for the reduction of various pollutants, including 2-nitro-phenol (2-NP), 2,6-dinitro-phenol (2,6-DNP), 4-nitro-phenol (4-NP), Congo red (CR), and methyl orange (MO) dyes with a strong sodium borohydride (NaBH4) reducing agent. The GL/Co-SnO2 nanocomposite synergistically reduced the MO in the presence of the reducing agent with greater reduction rate of 1.036 min-1 compared to other dyes. The reduction condition was optimized by changing various parameters, such as the catalyst amount, dye concentration, and the NaBH4 amount. Moreover, the GL/Co-SnO2 nanocomposite catalyst can be easily recovered, is recyclable, and revealed minimal loss of nanomaterials.
Keywords: Co-SnO2 nanomaterial; catalytic reduction; environmental remediation; gelatin hydrogel; recyclability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sorption of Organic Contaminants by Biopolymers: Role of Polarity, Structure and Domain Spatial Arrangement. [(accessed on 13 February 2020)]; Available online: https://www.ncbi.nlm.nih.gov/pubmed/17095043. - PubMed
-
- Bae H.J., Park H.J., Hong S.I., Byun Y.J., Darby D.O., Kimmel R.M., Whiteside W.S. Effect of clay content, homogenization RPM, pH, and ultrasonication on mechanical and barrier properties of fish gelatin/montmorillonite nanocomposite films. LWT Food Sci. Technol. 2009;42:1179–1186. doi: 10.1016/j.lwt.2008.12.016. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

