Thermosensitive Injectable Hydrogels for Intra-Articular Delivery of Etanercept for the Treatment of Osteoarthritis
- PMID: 36005089
- PMCID: PMC9407145
- DOI: 10.3390/gels8080488
Thermosensitive Injectable Hydrogels for Intra-Articular Delivery of Etanercept for the Treatment of Osteoarthritis
Abstract
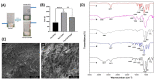
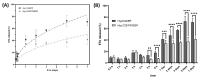
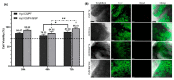
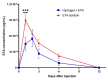
The intra-articular administration of drugs has attracted great interest in recent decades for the treatment of osteoarthritis. The use of modified drugs has also attracted interest in recent years because their intra-articular administration has demonstrated encouraging results. The objective of this work was to prepare injectable-thermosensitive hydrogels for the intra-articular administration of Etanercept (ETA), an inhibitor of tumor necrosis factor-α. Hydrogels were prepared from the physical mixture of chitosan and Pluronic F127 with β-glycerolphosphate (BGP). Adding β-glycerolphosphate to the system reduced the gelation time and also modified the morphology of the resulting material. In vitro studies were carried out to determine the cytocompatibility of the prepared hydrogels for the human chondrocyte line C28/I2. The in vitro release study showed that the incorporation of BGP into the system markedly modified the release of ETA. In the in vivo studies, it was verified that the hydrogels remained inside the implantation site in the joint until the end of the study. Furthermore, ETA was highly concentrated in the blood of the study mice 48 h after the loaded material was injected. Histological investigation of osteoarthritic knees showed that the material promotes cartilage recovery in osteoarthritic mice. The results demonstrate the potential of ETA-loaded injectable hydrogels for the localized treatment of joints.
Keywords: Pluronic; chitosan; etanercept; intra-articular delivery; osteoarthritis; thermosensitive hydrogels.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

