Somatostatin Receptor Theranostics for Refractory Meningiomas
- PMID: 36005176
- PMCID: PMC9406720
- DOI: 10.3390/curroncol29080438
Somatostatin Receptor Theranostics for Refractory Meningiomas
Abstract
Somatostatin receptor (SSTR)-targeted peptide receptor radionuclide therapy (PRRT) represents a promising approach for treatment-refractory meningiomas progressing after surgery and radiotherapy. The aim of this study was to provide outcomes of patients harboring refractory meningiomas treated by 177Lu-DOTATATE and an overall analysis of progression-free survival at 6 months (PFS-6) of the same relevant studies in the literature. Eight patients with recurrent and progressive WHO grade II meningiomas were treated after multimodal pretreatment with 177Lu-DOTATATE between 2019 and 2022. Primary and secondarily endpoints were progression-free survival at 6-months (PFS-6) and toxicity, respectively. PFS-6 analysis of our case series was compared with other similar relevant studies that included 86 patients treated with either 177Lu-DOTATATE or 90Y-DOTATOC. Our retrospective study showed a PFS-6 of 85.7% for WHO grade II progressive refractory meningiomas. Treatment was clinically and biologically well tolerated. The overall analysis of the previous relevant studies showed a PFS-6 of 89.7% for WHO grade I meningiomas (n = 29); 57.1% for WHO grade II (n = 21); and 0 % for WHO grade III (n = 12). For all grades (n = 86), including unknown grades, PFS-6 was 58.1%. SSTR-targeted PRRT allowed us to achieve prolonged PFS-6 in patients with WHO grade I and II progressive refractory meningiomas, except the most aggressive WHO grade II tumors. Large scale randomized trials are warranted for the better integration of PRRT in the treatment of refractory meningioma into clinical practice guidelines.
Keywords: meningioma; peptide receptor radionuclide therapy; somatostatin receptor; treatment-refractory meningioma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

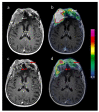
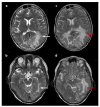
Similar articles
-
Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake.Neuro Oncol. 2016 Nov;18(11):1538-1547. doi: 10.1093/neuonc/now060. Epub 2016 Apr 21. Neuro Oncol. 2016. PMID: 27106404 Free PMC article.
-
Somatostatin Receptor-Targeted Radiopeptide Therapy in Treatment-Refractory Meningioma: Individual Patient Data Meta-analysis.J Nucl Med. 2021 Apr;62(4):507-513. doi: 10.2967/jnumed.120.249607. Epub 2020 Aug 28. J Nucl Med. 2021. PMID: 32859705
-
Long-term Efficacy, Survival, and Toxicity of Peptide Receptor Radionuclide Therapy in Patients With Refractory Meningioma.Clin Nucl Med. 2025 Jun 1;50(6):508-516. doi: 10.1097/RLU.0000000000005845. Epub 2025 May 5. Clin Nucl Med. 2025. PMID: 40320628
-
177Lu-labeled somatostatin receptor targeted radionuclide therapy dosimetry in meningioma: a systematic review.Q J Nucl Med Mol Imaging. 2024 Sep;68(3):217-225. doi: 10.23736/S1824-4785.24.03571-4. Epub 2024 Jul 19. Q J Nucl Med Mol Imaging. 2024. PMID: 39026463
-
Somatostatin Receptor Targeted PET-Imaging for Diagnosis, Radiotherapy Planning and Theranostics of Meningiomas: A Systematic Review of the Literature.Diagnostics (Basel). 2022 Jul 8;12(7):1666. doi: 10.3390/diagnostics12071666. Diagnostics (Basel). 2022. PMID: 35885570 Free PMC article. Review.
Cited by
-
Radioligand therapies in meningioma: Evidence and future directions.Neuro Oncol. 2024 Dec 9;26(Supplement_9):S215-S228. doi: 10.1093/neuonc/noae069. Neuro Oncol. 2024. PMID: 38702966 Free PMC article. Review.
-
A Narrative Review of Theranostics in Neuro-Oncology: Advancing Brain Tumor Diagnosis and Treatment Through Nuclear Medicine and Artificial Intelligence.Int J Mol Sci. 2025 Jul 31;26(15):7396. doi: 10.3390/ijms26157396. Int J Mol Sci. 2025. PMID: 40806525 Free PMC article. Review.
-
Peptide radionuclide radiation therapy with Lutathera in multirecurrent nonanaplastic meningiomas: antitumoral activity study by growth rate analysis.J Neurooncol. 2024 May;167(3):427-436. doi: 10.1007/s11060-024-04622-5. Epub 2024 Mar 7. J Neurooncol. 2024. PMID: 38451361
-
SSTR-directed peptide receptor radionuclide therapy for recurrent meningiomas: analysis of safety, efficacy and prognostic factors.Eur J Nucl Med Mol Imaging. 2025 Jun 2. doi: 10.1007/s00259-025-07336-6. Online ahead of print. Eur J Nucl Med Mol Imaging. 2025. PMID: 40455253
-
Semi-automated segmentation methods of SSTR PET for dosimetry prediction in refractory meningioma patients treated by SSTR-targeted peptide receptor radionuclide therapy.Eur Radiol. 2023 Oct;33(10):7089-7098. doi: 10.1007/s00330-023-09697-8. Epub 2023 May 6. Eur Radiol. 2023. PMID: 37148355 Review.
References
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

