Oral Fosfomycin Formulation in Bacterial Prostatitis: New Role for an Old Molecule-Brief Literature Review and Clinical Considerations
- PMID: 36005269
- PMCID: PMC9408554
- DOI: 10.3390/idr14040067
Oral Fosfomycin Formulation in Bacterial Prostatitis: New Role for an Old Molecule-Brief Literature Review and Clinical Considerations
Abstract
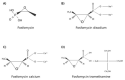
Bacterial prostatitis infections are described as infections that are difficult-to-treat, due to prostate anatomic characteristics along with clinical difficulty in terms of diagnosis and management. Furthermore, the emergence of multidrug resistant (MDR) bacteria, such as extended-spectrum beta-lactamase (ESBL) producer Escherichia coli, also representing the main causative pathogen in prostatitis, poses major problems in terms of antibiotic management and favorable clinical outcome. Oral fosfomycin, an antibiotic commonly used for the treatment of uncomplicated urinary tract infections (UTIs), has been recently evaluated for the treatment of bacterial prostatitis due to its favorable pharmacokinetic profile, its activity against MDR gram-positive and gram-negative bacteria, safety profile, and multiple synergic effect with other antibiotics as well as the low resistance rate. This review addresses fosfomycin pharmacokinetics and pharmacodynamics and discusses the latest clinical evidence on its clinical use to treat acute and chronic bacterial prostatitis in hospitalized patients and in outpatients. As described in several reports, oral fosfomycin may represent a valid therapeutic option to treat susceptible germs commonly causing prostatitis, such as E. coli and other Enterobacterales as well as Enterococcus faecium, even as a first-line regimen in particular clinical settings (patients with previous treatment failure, with allergies or outpatients). Stronger data from further studies, including randomized controlled trials, would be helpful to establish the proper dosage and specific indications.
Keywords: bacterial prostatitis; oral fosfomycin; urinary tract infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- NIH Consensus Definition and Classification of Prostatitis|JAMA|JAMA Network. [(accessed on 4 July 2022)]. Available online: https://jamanetwork.com/journals/jama/article-abstract/1030245.
-
- Erdem H., Hargreaves S., Ankarali H., Caskurlu H., Ceviker S.A., Bahar-Kacmaz A., Meric-Koc M., Altindis M., Yildiz-Kirazaldi Y., Kizilates F., et al. Managing adult patients with infectious diseases in emergency departments: International ID-IRI study. J. Chemother. 2021;33:302–318. doi: 10.1080/1120009X.2020.1863696. - DOI - PubMed
-
- El-Sokkary R., Uysal S., Erdem H., Kullar R., Pekok A.U., Amer F., Grgić S., Carevic B., El-Kholy A., Liskova A., et al. Profiles of multidrug-resistant organisms among patients with bacteremia in intensive care units: An international ID-IRI survey. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:2323–2334. doi: 10.1007/s10096-021-04288-1. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

