Epidemiological aspects of headache after different types of COVID-19 vaccines: An online survey
- PMID: 36005277
- PMCID: PMC9538602
- DOI: 10.1111/head.14374
Epidemiological aspects of headache after different types of COVID-19 vaccines: An online survey
Abstract
Background: Coronavirus disease 2019 (COVID-19) vaccine-related side effects are a key concern with the emergence of various types of vaccines in the market. We aimed to assess the frequency and characteristics of headache following different types of COVID-19 vaccines.
Methods: Fully vaccinated people were recruited by a convenience sample through an online survey from September 1 to December 1, 2021. Detailed analysis of headache following vaccination was investigated. Participants with a history of pre-existing headaches were telephone interviewed by a neurologist to ascertain the type of headache.
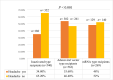
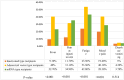
Results: A total of 1372 participants participated (mean age 32.9 ± 11.1). The highest frequency of headache was reported with the adenoviral vector type (302/563, 53.6%), followed by mRNA vaccines (129/269, 48%) and then the inactivated type (188/540, 34.8%). Recipients of the adenoviral vector type had a significantly longer latency between vaccination and the headache onset (median 8 h [5:12]) than recipients of the inactivated type (median 4 h [2:8], p < 0.001). Headache intensity was significantly higher with the adenoviral vector type (median 6 [5:8]) than with the inactivated type (median 5 [4:7], p < 0.001). Adenoviral vector vaccines would increase the likelihood of headache by 2.38 times more than inactivated vaccines (odds ratio [OR] 2.38, 95% confidence interval [CI] 1.83-3.04, p < 0.001). Female sex and thyroid disease were significantly associated with headache related to COVID-19 vaccines (OR 1.52, 95% CI 1.16-1.99; OR 3.97, 95% CI 1.55-10.2, respectively).
Conclusion: Recipients of the COVID-19 vaccine should be counseled that they may experience headaches, especially after the adenoviral vector type. However, the intensity of such headache is mild to moderate and can resolve within a few days. Based on the current study design and the potential recall bias, these results may not be generalizable and should be preliminary.
Keywords: COVID-19 vaccines; adenoviral vector vaccine; headache; inactivated vaccine; mRNA-based vaccine.
© 2022 American Headache Society.
Conflict of interest statement
All authors had no disclosures.
Figures
Similar articles
-
Reported COVID-19 vaccines side effects among Algerian athletes: a comparison between inactivated virus, adenoviral vector, and mRNA COVID-19 vaccines.Phys Sportsmed. 2024 Apr;52(2):134-146. doi: 10.1080/00913847.2023.2186691. Epub 2023 Mar 6. Phys Sportsmed. 2024. PMID: 36876437
-
The characteristics of COVID-19 vaccine-related headache: Clues gathered from the healthcare personnel in the pandemic.Cephalalgia. 2022 Apr;42(4-5):366-375. doi: 10.1177/03331024211042390. Epub 2021 Sep 12. Cephalalgia. 2022. PMID: 34510919 Free PMC article.
-
Reporting adverse events of COVID-19 vaccines: The case of Bulgaria.PLoS One. 2022 Jun 10;17(6):e0269727. doi: 10.1371/journal.pone.0269727. eCollection 2022. PLoS One. 2022. PMID: 35687609 Free PMC article.
-
Headache following vaccination against COVID-19 among healthcare workers with a history of COVID-19 infection: a cross-sectional study in Iran with a meta-analytic review of the literature.Head Face Med. 2023 May 19;19(1):19. doi: 10.1186/s13005-023-00363-4. Head Face Med. 2023. PMID: 37202794 Free PMC article. Review.
-
Headaches and facial pain attributed to SARS-CoV-2 infection and vaccination: a systematic review.Eur J Neurol. 2024 Jun;31(6):e16251. doi: 10.1111/ene.16251. Epub 2024 Feb 28. Eur J Neurol. 2024. PMID: 38415282 Free PMC article.
Cited by
-
COVID-19 vaccination-related headache showed two different clusters in the long-term course: a prospective multicenter follow-up study (COVA-Head Study).J Headache Pain. 2023 Sep 29;24(1):132. doi: 10.1186/s10194-023-01665-3. J Headache Pain. 2023. PMID: 37773092 Free PMC article.
-
Differences and similarities between COVID-19 related-headache and COVID-19 vaccine related-headache. A case-control study.Rev Neurol. 2023 Nov 16;77(10):229-239. doi: 10.33588/rn.7710.2023063. Rev Neurol. 2023. PMID: 37962534 Free PMC article. English, Spanish.
References
-
- Krammer F. SARS‐CoV‐2 vaccines in development. Nature. 2020;586:516‐527. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

