Functional Role of AsAP in the Reproduction of Adelphocoris suturalis (Hemiptera: Miridae)
- PMID: 36005380
- PMCID: PMC9409435
- DOI: 10.3390/insects13080755
Functional Role of AsAP in the Reproduction of Adelphocoris suturalis (Hemiptera: Miridae)
Abstract
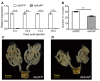
Adelphocoris suturalis Jakovlev (Hemiptera: Miridae) is an omnivorous agricultural pest that has severe economic impacts on a diverse range of agricultural crops. Although the targeted disruption of reproductive development among insects has been proposed as a novel control strategy for pest species, the current understanding of the physiology and molecular mechanisms of A. suturalis reproduction is very limited. In this study, we isolated a putative A. suturalisaspartic protease (AsAP) gene that is highly expressed in the fat body and ovaries of sexually mature females. The double-stranded RNA (dsRNA)-mediated knockdown of AsAP suppressed ovarian development and negatively impacted female fertility, which suggested that it plays an essential role in A. suturalis reproduction. The results of this study could help to expand our understanding of A. suturalis reproductive development and have the potential to facilitate the development of effective strategies for the better control of this pest species.
Keywords: Adelphocoris suturalis Jakovlev; RNAi; aspartic protease; ovarian development; reproduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Huis A.V. The Global Impact of Insects. Wageningen UR; Wageningen, The Netherlands: 2014.
Grants and funding
LinkOut - more resources
Full Text Sources

