Toxin-Producing Endosymbionts Shield Pathogenic Fungus against Micropredators
- PMID: 36005392
- PMCID: PMC9600703
- DOI: 10.1128/mbio.01440-22
Toxin-Producing Endosymbionts Shield Pathogenic Fungus against Micropredators
Abstract
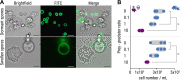
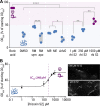
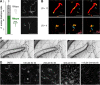
The fungus Rhizopus microsporus harbors a bacterial endosymbiont (Mycetohabitans rhizoxinica) for the production of the antimitotic toxin rhizoxin. Although rhizoxin is the causative agent of rice seedling blight, the toxinogenic bacterial-fungal alliance is, not restricted to the plant disease. It has been detected in numerous environmental isolates from geographically distinct sites covering all five continents, thus raising questions regarding the ecological role of rhizoxin beyond rice seedling blight. Here, we show that rhizoxin serves the fungal host in fending off protozoan and metazoan predators. Fluorescence microscopy and coculture experiments with the fungivorous amoeba Protostelium aurantium revealed that ingestion of R. microsporus spores is toxic to P. aurantium. This amoebicidal effect is caused by the dominant bacterial rhizoxin congener rhizoxin S2, which is also lethal toward the model nematode Caenorhabditis elegans. By combining stereomicroscopy, automated image analysis, and quantification of nematode movement, we show that the fungivorous nematode Aphelenchus avenae actively feeds on R. microsporus that is lacking endosymbionts, whereas worms coincubated with symbiotic R. microsporus are significantly less lively. This study uncovers an unexpected ecological role of rhizoxin as shield against micropredators. This finding suggests that predators may function as an evolutionary driving force to maintain toxin-producing endosymbionts in nonpathogenic fungi. IMPORTANCE The soil community is a complex system characterized by predator-prey interactions. Fungi have developed effective strategies to defend themselves against predators. Understanding these strategies is of critical importance for ecology, medicine, and biotechnology. In this study, we shed light on the defense mechanisms of the phytopathogenic Rhizopus-Mycetohabitans symbiosis that has spread worldwide. We report an unexpected role of rhizoxin, a secondary metabolite produced by the bacterium M. rhizoxinica residing within the hyphae of R. microsporus. We show that this bacterial secondary metabolite is utilized by the fungal host to successfully fend off fungivorous protozoan and metazoan predators and thus identified a fundamentally new function of this infamous cytotoxic compound. This endosymbiont-dependent predator defense illustrates an unusual strategy employed by fungi that has broader implications, since it may serve as a model for understanding how animal predation acts as an evolutionary driving force to maintain endosymbionts in nonpathogenic fungi.
Keywords: Rhizopus; microbial ecology; microbial interactions; natural products; rhizoxin; secondary metabolism; symbiosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Antimitotic rhizoxin derivatives from a cultured bacterial endosymbiont of the rice pathogenic fungus Rhizopus microsporus.J Am Chem Soc. 2006 Sep 6;128(35):11529-36. doi: 10.1021/ja062953o. J Am Chem Soc. 2006. PMID: 16939276
-
Evolution of an endofungal lifestyle: Deductions from the Burkholderia rhizoxinica genome.BMC Genomics. 2011 May 4;12:210. doi: 10.1186/1471-2164-12-210. BMC Genomics. 2011. PMID: 21539752 Free PMC article.
-
Evolution of host resistance in a toxin-producing bacterial-fungal alliance.ISME J. 2008 Jun;2(6):632-41. doi: 10.1038/ismej.2008.19. Epub 2008 Feb 28. ISME J. 2008. PMID: 18309361
-
Insect pathogens as biological control agents: Back to the future.J Invertebr Pathol. 2015 Nov;132:1-41. doi: 10.1016/j.jip.2015.07.009. Epub 2015 Jul 27. J Invertebr Pathol. 2015. PMID: 26225455 Review.
-
Pulmonary defense mechanisms against opportunistic fungal pathogens.Immunol Ser. 1989;47:243-71. Immunol Ser. 1989. PMID: 2490078 Review.
Cited by
-
Deazaflavin metabolite produced by endosymbiotic bacteria controls fungal host reproduction.ISME J. 2024 Jan 8;18(1):wrae074. doi: 10.1093/ismejo/wrae074. ISME J. 2024. PMID: 38691425 Free PMC article.
-
Alternative in-vivo models of mucormycosis.Front Cell Infect Microbiol. 2024 Feb 1;14:1343834. doi: 10.3389/fcimb.2024.1343834. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38362495 Free PMC article. Review.
-
Genomic evidence of symbiotic adaptations in fungus-associated bacteria.iScience. 2025 Mar 20;28(4):112253. doi: 10.1016/j.isci.2025.112253. eCollection 2025 Apr 18. iScience. 2025. PMID: 40290873 Free PMC article.
-
Comparative genomics of Mollicutes-related endobacteria supports a late invasion into Mucoromycota fungi.Commun Biol. 2023 Sep 18;6(1):948. doi: 10.1038/s42003-023-05299-8. Commun Biol. 2023. PMID: 37723238 Free PMC article.
-
Genomic insights reveal community structure and phylogenetic associations of endohyphal bacteria and viruses in fungal endophytes.Environ Microbiome. 2025 Jul 25;20(1):95. doi: 10.1186/s40793-025-00757-8. Environ Microbiome. 2025. PMID: 40713930 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

