Vascular Implications of COVID-19: Role of Radiological Imaging, Artificial Intelligence, and Tissue Characterization: A Special Report
- PMID: 36005433
- PMCID: PMC9409845
- DOI: 10.3390/jcdd9080268
Vascular Implications of COVID-19: Role of Radiological Imaging, Artificial Intelligence, and Tissue Characterization: A Special Report
Abstract
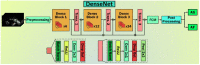
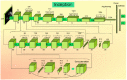
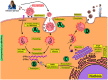
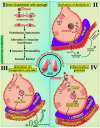
The SARS-CoV-2 virus has caused a pandemic, infecting nearly 80 million people worldwide, with mortality exceeding six million. The average survival span is just 14 days from the time the symptoms become aggressive. The present study delineates the deep-driven vascular damage in the pulmonary, renal, coronary, and carotid vessels due to SARS-CoV-2. This special report addresses an important gap in the literature in understanding (i) the pathophysiology of vascular damage and the role of medical imaging in the visualization of the damage caused by SARS-CoV-2, and (ii) further understanding the severity of COVID-19 using artificial intelligence (AI)-based tissue characterization (TC). PRISMA was used to select 296 studies for AI-based TC. Radiological imaging techniques such as magnetic resonance imaging (MRI), computed tomography (CT), and ultrasound were selected for imaging of the vasculature infected by COVID-19. Four kinds of hypotheses are presented for showing the vascular damage in radiological images due to COVID-19. Three kinds of AI models, namely, machine learning, deep learning, and transfer learning, are used for TC. Further, the study presents recommendations for improving AI-based architectures for vascular studies. We conclude that the process of vascular damage due to COVID-19 has similarities across vessel types, even though it results in multi-organ dysfunction. Although the mortality rate is ~2% of those infected, the long-term effect of COVID-19 needs monitoring to avoid deaths. AI seems to be penetrating the health care industry at warp speed, and we expect to see an emerging role in patient care, reduce the mortality and morbidity rate.
Keywords: COVID-19; artificial intelligence; carotid; coronary; coronavirus; pulmonary; renal; vascular damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


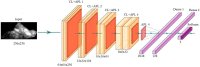
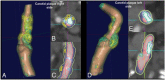
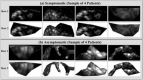
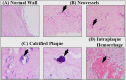
References
-
- Baschi R., Luca A., Nicoletti A., Caccamo M., Cicero C.E., D’Agate C., Di Giorgi L., La Bianca G., Lo Castro T., Zappia M. Changes in motor, cognitive, and behavioral symptoms in Parkinson’s disease and mild cognitive impairment during the COVID-19 lockdown. Front. Psychiatry. 2020;11:590134. doi: 10.3389/fpsyt.2020.590134. - DOI - PMC - PubMed
-
- Suri J.S., Puvvula A., Biswas M., Majhail M., Saba L., Faa G., Singh I.M., Oberleitner R., Turk M., Chadha P.S. COVID-19 pathways for brain and heart injury in comorbidity patients: A role of medical imaging and artificial intelligence-based COVID severity classification: A review. Comput. Biol. Med. 2020;124:103960. doi: 10.1016/j.compbiomed.2020.103960. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

