Discovery of AZD4831, a Mechanism-Based Irreversible Inhibitor of Myeloperoxidase, As a Potential Treatment for Heart Failure with Preserved Ejection Fraction
- PMID: 36005476
- PMCID: PMC9469207
- DOI: 10.1021/acs.jmedchem.1c02141
Discovery of AZD4831, a Mechanism-Based Irreversible Inhibitor of Myeloperoxidase, As a Potential Treatment for Heart Failure with Preserved Ejection Fraction
Erratum in
-
Correction to "Discovery of AZD4831, a Mechanism-Based Irreversible Inhibitor of Myeloperoxidase, As a Potential Treatment for Heart Failure with Preserved Ejection Fraction".J Med Chem. 2022 Oct 13;65(19):13482. doi: 10.1021/acs.jmedchem.2c01533. Epub 2022 Oct 3. J Med Chem. 2022. PMID: 36190771 Free PMC article. No abstract available.
Abstract
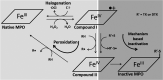
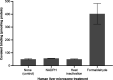
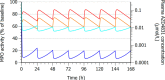
Myeloperoxidase is a promising therapeutic target for treatment of patients suffering from heart failure with preserved ejection fraction (HFpEF). We aimed to discover a covalent myeloperoxidase inhibitor with high selectivity for myeloperoxidase over thyroid peroxidase, limited penetration of the blood-brain barrier, and pharmacokinetics suitable for once-daily oral administration at low dose. Structure-activity relationship, biophysical, and structural studies led to prioritization of four compounds for in-depth safety and pharmacokinetic studies in animal models. One compound (AZD4831) progressed to clinical studies on grounds of high potency (IC50, 1.5 nM in vitro) and selectivity (>450-fold vs thyroid peroxidase in vitro), the mechanism of irreversible inhibition, and the safety profile. Following phase 1 studies in healthy volunteers and a phase 2a study in patients with HFpEF, a phase 2b/3 efficacy study of AZD4831 in patients with HFpEF started in 2021.
Conflict of interest statement
The authors declare the following competing financial interest(s): All authors are employees of AstraZeneca and own stock or stock options.
Figures



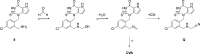



Similar articles
-
Myeloperoxidase Inhibition in Heart Failure With Preserved or Mildly Reduced Ejection Fraction: SATELLITE Trial Results.J Card Fail. 2024 Jan;30(1):104-110. doi: 10.1016/j.cardfail.2023.04.003. Epub 2023 Apr 16. J Card Fail. 2024. PMID: 37072105 Clinical Trial.
-
Early Clinical Experience With AZD4831, A Novel Myeloperoxidase Inhibitor, Developed for Patients With Heart Failure With Preserved Ejection Fraction.Clin Transl Sci. 2021 May;14(3):812-819. doi: 10.1111/cts.12859. Epub 2021 May 2. Clin Transl Sci. 2021. PMID: 32770730 Free PMC article. Clinical Trial.
-
Myeloperoxidase Inhibition Reverses Biomarker Profiles Associated With Clinical Outcomes in HFpEF.JACC Heart Fail. 2023 Jul;11(7):775-787. doi: 10.1016/j.jchf.2023.03.002. Epub 2023 May 3. JACC Heart Fail. 2023. PMID: 37140510 Clinical Trial.
-
A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation.J Am Coll Cardiol. 2013 Jul 23;62(4):263-71. doi: 10.1016/j.jacc.2013.02.092. Epub 2013 May 15. J Am Coll Cardiol. 2013. PMID: 23684677 Review.
-
Drugs That Ameliorate Epicardial Adipose Tissue Inflammation May Have Discordant Effects in Heart Failure With a Preserved Ejection Fraction as Compared With a Reduced Ejection Fraction.J Card Fail. 2019 Dec;25(12):986-1003. doi: 10.1016/j.cardfail.2019.09.002. Epub 2019 Sep 18. J Card Fail. 2019. PMID: 31541742 Review.
Cited by
-
Role of Myeloperoxidase, Oxidative Stress, and Inflammation in Bronchopulmonary Dysplasia.Antioxidants (Basel). 2024 Jul 23;13(8):889. doi: 10.3390/antiox13080889. Antioxidants (Basel). 2024. PMID: 39199135 Free PMC article. Review.
-
Targeting myeloperoxidase limits myeloid cell immunosuppression enhancing immune checkpoint therapy for pancreatic cancer.Cancer Immunol Immunother. 2024 Feb 17;73(3):57. doi: 10.1007/s00262-024-03647-z. Cancer Immunol Immunother. 2024. PMID: 38367056 Free PMC article.
-
Rationale and design of ENDEAVOR: A sequential phase 2b-3 randomized clinical trial to evaluate the effect of myeloperoxidase inhibition on symptoms and exercise capacity in heart failure with preserved or mildly reduced ejection fraction.Eur J Heart Fail. 2023 Sep;25(9):1696-1707. doi: 10.1002/ejhf.2977. Epub 2023 Aug 22. Eur J Heart Fail. 2023. PMID: 37470101 Free PMC article. Clinical Trial.
-
Reacon: a template- and cluster-based framework for reaction condition prediction.Chem Sci. 2024 Dec 6;16(2):854-866. doi: 10.1039/d4sc05946h. eCollection 2025 Jan 2. Chem Sci. 2024. PMID: 39650221 Free PMC article.
-
The myeloperoxidase inhibitor mitiperstat (AZD4831) does not prolong the QT interval at expected therapeutic doses.Pharmacol Res Perspect. 2024 Apr;12(2):e1184. doi: 10.1002/prp2.1184. Pharmacol Res Perspect. 2024. PMID: 38445541 Free PMC article. Clinical Trial.
References
-
- Hage C.; Michaelsson E.; Kull B.; Miliotis T.; Svedlund S.; Linde C.; Donal E.; Daubert J. C.; Gan L. M.; Lund L. H. Myeloperoxidase and related biomarkers are suggestive footprints of endothelial microvascular inflammation in HFpEF patients. ESC Heart Fail 2020, 7 (4), 1534–1546. 10.1002/ehf2.12700. - DOI - PMC - PubMed
-
- Shah S. J.; Lam C. S. P.; Svedlund S.; Saraste A.; Hage C.; Tan R. S.; Beussink-Nelson L.; Ljung Faxen U.; Fermer M. L.; Broberg M. A.; Gan L. M.; Lund L. H. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39 (37), 3439–3450. 10.1093/eurheartj/ehy531. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases
Research Materials

