Cancer-associated fibroblast-released extracellular vesicles carrying miR-199a-5p induces the progression of gastric cancer through regulation of FKBP5-mediated AKT1/mTORC1 signaling pathway
- PMID: 36005478
- PMCID: PMC9704384
- DOI: 10.1080/15384101.2022.2105092
Cancer-associated fibroblast-released extracellular vesicles carrying miR-199a-5p induces the progression of gastric cancer through regulation of FKBP5-mediated AKT1/mTORC1 signaling pathway
Abstract
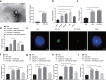
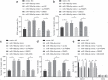
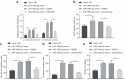
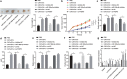
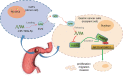
Accumulating evidence has unfolded the significance of extracellular vesicles (EVs) in diseases and cancers. Here, we attempted to discuss the role of cancer-associated fibroblasts (CAFs)-derived EVs containing miR-199a-5p in gastric tumorigenesis. Upregulated miR-199a-5p was first identified in cancer cells. Then, we selected CAFs for isolation of EVs which were co-cultured with AGS cells. We observed successful delivery of miR-199a-5p via CAF-derived EVs. Besides, miR-199a-5p promoted malignant properties of AGS cells. Moreover, miR-199a-5p downregulated FKBP5, leading to upregulated phosphorylation level of AKT1, which promoted the malignant phenotypes of AGS cells by activating mammalian target of rapamycin complex 1(mTORC1). Exosomal miR-199a-5p from CAFs promoted gastric tumorigenesis in vivo. Our findings point toward the critical role of CAFs-derived EVs carrying miR-199a-5p in gastric cancer progression.
Keywords: AKT1; Cancer-associated fibroblasts; FKBP5; extracellular vesicles; gastric cancer; mTORC1; microRNA-199a-5p.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Extracellular vesicles-encapsulated microRNA-10a-5p shed from cancer-associated fibroblast facilitates cervical squamous cell carcinoma cell angiogenesis and tumorigenicity via Hedgehog signaling pathway.Cancer Gene Ther. 2021 May;28(5):529-542. doi: 10.1038/s41417-020-00238-9. Epub 2020 Nov 24. Cancer Gene Ther. 2021. PMID: 33235271
-
Cancer-associated fibroblasts derived extracellular vesicles promote angiogenesis of colorectal adenocarcinoma cells through miR-135b-5p/FOXO1 axis.Cancer Biol Ther. 2022 Dec 31;23(1):76-88. doi: 10.1080/15384047.2021.2017222. Cancer Biol Ther. 2022. PMID: 35100092 Free PMC article.
-
Breast cancer cell-derived extracellular vesicles transfer miR-182-5p and promote breast carcinogenesis via the CMTM7/EGFR/AKT axis.Mol Med. 2021 Jul 16;27(1):78. doi: 10.1186/s10020-021-00338-8. Mol Med. 2021. PMID: 34294040 Free PMC article.
-
Extracellular vesicles derived from cancer-associated fibroblast carries miR-224-5p targeting SLC4A4 to promote the proliferation, invasion and migration of colorectal cancer cells.Carcinogenesis. 2021 Oct 5;42(9):1143-1153. doi: 10.1093/carcin/bgab055. Carcinogenesis. 2021. PMID: 34170291
-
Current progress in microRNA profiling of circulating extracellular vesicles in amyotrophic lateral sclerosis: A systematic review.Neurobiol Dis. 2024 Oct 1;200:106639. doi: 10.1016/j.nbd.2024.106639. Epub 2024 Aug 19. Neurobiol Dis. 2024. PMID: 39168358
Cited by
-
The role of microRNAs in the gastric cancer tumor microenvironment.Mol Cancer. 2024 Aug 20;23(1):170. doi: 10.1186/s12943-024-02084-x. Mol Cancer. 2024. PMID: 39164671 Free PMC article. Review.
-
Regulatory roles of ncRNAs in gastric cancer progression: a comprehensive insight into key signaling pathways.Med Oncol. 2025 Jul 1;42(8):309. doi: 10.1007/s12032-025-02828-9. Med Oncol. 2025. PMID: 40593327 Review.
-
piRNAs may regulate expression of candidate genes of esophageal adenocarcinoma.Front Genet. 2022 Nov 30;13:1069637. doi: 10.3389/fgene.2022.1069637. eCollection 2022. Front Genet. 2022. PMID: 36531220 Free PMC article.
-
Cancer-Associated Fibroblast-Secreted Exosomes Promote Gastric Cancer Cell Migration and Invasion via the IL-32/ESR1 Axis.Appl Biochem Biotechnol. 2024 Sep;196(9):6045-6058. doi: 10.1007/s12010-023-04782-6. Epub 2024 Jan 5. Appl Biochem Biotechnol. 2024. PMID: 38180644
-
Gastroesophageal circulating tumor cell crosstalk with peripheral immune system guides CTC survival and proliferation.Cell Death Dis. 2025 Mar 29;16(1):223. doi: 10.1038/s41419-025-07530-2. Cell Death Dis. 2025. PMID: 40157906 Free PMC article.
References
-
- Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388(10060):2654–2664. - PubMed
-
- Ajani JA, Lee J, Sano T, et al. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036. - PubMed
-
- Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet. 2020;396(10251):635–648. - PubMed
-
- Johnston FM, Beckman M.. Updates on management of gastric cancer. Curr Oncol Rep. 2019;21(8):67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
