A Novel Marine Pyran-Isoindolone Compound Enhances Fibrin Lysis Mediated by Single-Chain Urokinase-Type Plasminogen Activator
- PMID: 36005498
- PMCID: PMC9410493
- DOI: 10.3390/md20080495
A Novel Marine Pyran-Isoindolone Compound Enhances Fibrin Lysis Mediated by Single-Chain Urokinase-Type Plasminogen Activator
Abstract
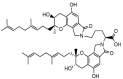
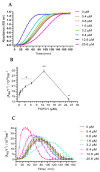
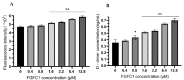
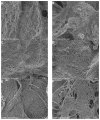
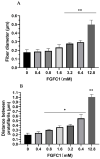
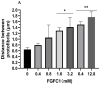
Fungi fibrinolytic compound 1 (FGFC1) is a rare pyran-isoindolone derivative with fibrinolytic activity. The aim of this study was to further determine the effect of FGFC1 on fibrin clots lysis in vitro. We constructed a fibrinolytic system containing single-chain urokinase-type plasminogen activator (scu-PA) and plasminogen to measure the fibrinolytic activity of FGFC1 using the chromogenic substrate method. After FITC-fibrin was incubated with increasing concentrations of FGFC1, the changes in the fluorescence intensity and D-dimer in the lysate were measured using a fluorescence microplate reader. The fibrin clot structure induced by FGFC1 was observed and analyzed using a scanning electron microscope and laser confocal microscope. We found that the chromogenic reaction rate of the mixture system increased from (15.9 ± 1.51) × 10−3 min−1 in the control group to (29.7 ± 1.25) × 10−3 min−1 for 12.8 μM FGFC1(p < 0.01). FGFC1 also significantly increased the fluorescence intensity and d-dimer concentration in FITC fibrin lysate. Image analysis showed that FGFC1 significantly reduced the fiber density and increased the fiber diameter and the distance between protofibrils. These results show that FGFC1 can effectively promote fibrin lysis in vitro and may represent a novel candidate agent for thrombolytic therapy.
Keywords: FGFC1; fibrin clot; fibrinolysis; fibrinolytic activity; plasminogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation. 2019;139:E56–E528. doi: 10.1161/CIR.0000000000000659. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

