Marine Arthropods as a Source of Antimicrobial Peptides
- PMID: 36005504
- PMCID: PMC9409781
- DOI: 10.3390/md20080501
Marine Arthropods as a Source of Antimicrobial Peptides
Abstract
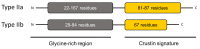
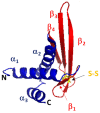
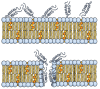
Peptide therapeutics play a key role in the development of new medical treatments. The traditional focus on endogenous peptides has shifted from first discovering other natural sources of these molecules, to later synthesizing those with unique bioactivities. This review provides concise information concerning antimicrobial peptides derived from marine crustaceans for the development of new therapeutics. Marine arthropods do not have an adaptive immune system, and therefore, they depend on the innate immune system to eliminate pathogens. In this context, antimicrobial peptides (AMPs) with unique characteristics are a pivotal part of the defense systems of these organisms. This review covers topics such as the diversity and distribution of peptides in marine arthropods (crustacea and chelicerata), with a focus on penaeid shrimps. The following aspects are covered: the defense system; classes of AMPs; molecular characteristics of AMPs; AMP synthesis; the role of penaeidins, anti-lipopolysaccharide factors, crustins, and stylicins against microorganisms; and the use of AMPs as therapeutic drugs. This review seeks to provide a useful compilation of the most recent information regarding AMPs from marine crustaceans, and describes the future potential applications of these molecules.
Keywords: AMP; antimicrobial peptides; crustaceans; defense system; drug; shrimp.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



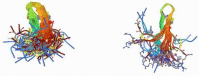
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

