Biochemical Characterization and Elucidation of the Hybrid Action Mode of a New Psychrophilic and Cold-Tolerant Alginate Lyase for Efficient Preparation of Alginate Oligosaccharides
- PMID: 36005509
- PMCID: PMC9410210
- DOI: 10.3390/md20080506
Biochemical Characterization and Elucidation of the Hybrid Action Mode of a New Psychrophilic and Cold-Tolerant Alginate Lyase for Efficient Preparation of Alginate Oligosaccharides
Abstract
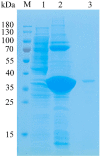
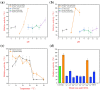
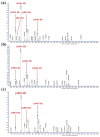
Alginate lyases with unique biochemical properties have irreplaceable value in food and biotechnology industries. Herein, the first new hybrid action mode Thalassotalea algicola-derived alginate lyase gene (TAPL7A) with both psychrophilic and cold-tolerance was cloned and expressed heterologously in E. coli. With the highest sequence identity (43%) to the exolytic alginate lyase AlyA5 obtained from Zobellia galactanivorans, TAPL7A was identified as a new polysaccharide lyases family 7 (PL7) alginate lyase. TAPL7A has broad substrate tolerance with specific activities of 4186.1 U/mg, 2494.8 U/mg, 2314.9 U/mg for polyM, polyG, and sodium alginate, respectively. Biochemical characterization of TAPL7A showed optimal activity at 15 °C, pH 8.0. Interestingly, TAPL7A exhibits both extreme psychrophilic and cold tolerance, which other cold-adapted alginate lyase do not possess. In a wide range of 5-30 °C, the activity can reach 80-100%, and the residual activity of more than 70% can still be maintained after 1 h of incubation. Product analysis showed that TAPL7A adopts a hybrid endo/exo-mode on all three substrates. FPLC and ESI-MS confirmed that the final products of TAPL7A are oligosaccharides with degrees of polymerization (Dps) of 1-2. This study provides excellent alginate lyase candidates for low-temperature environmental applications in food, agriculture, medicine and other industries.
Keywords: alginate lyase; biochemical characterization; cold-tolerant; endo/exolytic; psychrophilic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Biochemical characterization and elucidation the degradation pattern of a new cold-adapted and Ca2+ activated alginate lyase for efficient preparation of alginate oligosaccharides.Enzyme Microb Technol. 2023 Jan;162:110146. doi: 10.1016/j.enzmictec.2022.110146. Epub 2022 Oct 19. Enzyme Microb Technol. 2023. PMID: 36279637
-
Characterization of a New Biofunctional, Exolytic Alginate Lyase from Tamlana sp. s12 with High Catalytic Activity and Cold-Adapted Features.Mar Drugs. 2021 Mar 28;19(4):191. doi: 10.3390/md19040191. Mar Drugs. 2021. PMID: 33800691 Free PMC article.
-
A Novel Cold-Adapted and High-Alkaline Alginate Lyase with Potential for Alginate Oligosaccharides Preparation.Molecules. 2023 Aug 22;28(17):6190. doi: 10.3390/molecules28176190. Molecules. 2023. PMID: 37687019 Free PMC article.
-
Recent progress on engineering microbial alginate lyases towards their versatile role in biotechnological applications.Folia Microbiol (Praha). 2020 Dec;65(6):937-954. doi: 10.1007/s12223-020-00802-8. Epub 2020 Jun 4. Folia Microbiol (Praha). 2020. PMID: 32500437 Review.
-
Alginate degrading enzymes: an updated comprehensive review of the structure, catalytic mechanism, modification method and applications of alginate lyases.Crit Rev Biotechnol. 2021 Sep;41(6):953-968. doi: 10.1080/07388551.2021.1898330. Epub 2021 May 20. Crit Rev Biotechnol. 2021. PMID: 34015998 Review.
Cited by
-
Characterization and Mechanism Study of a Novel PL7 Family Exolytic Alginate Lyase from Marine Bacteria Vibrio sp. W13.Appl Biochem Biotechnol. 2024 Jan;196(1):68-84. doi: 10.1007/s12010-023-04483-0. Epub 2023 Apr 26. Appl Biochem Biotechnol. 2024. PMID: 37099125
-
Characterization of bifunctional alginate lyase Aly644 and antimicrobial activity of enzymatic hydrolysates.Appl Microbiol Biotechnol. 2023 Nov;107(22):6845-6857. doi: 10.1007/s00253-023-12745-4. Epub 2023 Sep 12. Appl Microbiol Biotechnol. 2023. PMID: 37698609
-
Efficient Degradation of Alginate and Preparation of Alginate Oligosaccharides by a Novel Biofunctional Alginate Lyase with High Activity and Excellent Thermophilic Features.Mar Drugs. 2023 Mar 14;21(3):180. doi: 10.3390/md21030180. Mar Drugs. 2023. PMID: 36976229 Free PMC article.
References
-
- Wang Y., Han F., Hu B., Li J., Yu W. In vivo Prebiotic Properties of Alginate Oligosaccharides Prepared Through Enzymatic Hydrolysis of Alginate. Nutr. Res. 2006;26:597–603. doi: 10.1016/j.nutres.2006.09.015. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

