Antifouling Marine Coatings with a Potentially Safer and Sustainable Synthetic Polyphenolic Derivative
- PMID: 36005510
- PMCID: PMC9409691
- DOI: 10.3390/md20080507
Antifouling Marine Coatings with a Potentially Safer and Sustainable Synthetic Polyphenolic Derivative
Abstract
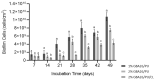
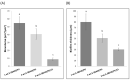
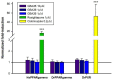
The development of harmless substances to replace biocide-based coatings used to prevent or manage marine biofouling and its unwanted consequences is urgent. The formation of biofilms on submerged marine surfaces is one of the first steps in the marine biofouling process, which facilitates the further settlement of macrofoulers. Anti-biofilm properties of a synthetic polyphenolic compound, with previously described anti-settlement activity against macrofoulers, were explored in this work. In solution this new compound was able to prevent biofilm formation and reduce a pre-formed biofilm produced by the marine bacterium, Pseudoalteromonas tunicata. Then, this compound was applied to a marine coating and the formation of P. tunicata biofilms was assessed under hydrodynamic conditions to mimic the marine environment. For this purpose, polyurethane (PU)-based coating formulations containing 1 and 2 wt.% of the compound were prepared based on a prior developed methodology. The most effective formulation in reducing the biofilm cell number, biovolume, and thickness was the PU-based coating containing an aziridine-based crosslinker and 2 wt.% of the compound. To assess the marine ecotoxicity impact of this compound, its potential to disrupt endocrine processes was evaluated through the modulation of two nuclear receptors (NRs), peroxisome proliferator-activated receptor γ (PPARγ), and pregnane X receptor (PXR). Transcriptional activation of the selected NRs upon exposure to the polyphenolic compound (10 µM) was not observed, thus highlighting the eco-friendliness towards the addressed NRs of this new dual-acting anti-macro- and anti-microfouling agent towards the addressed NRs.
Keywords: anti-biofilm; endocrine disruptor assessment; gallic acid; marine bacteria; safer chemicals.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: antifouling compound, method, and uses thereof to INPI #PT 117494: A.R. Neves, C. Vilas-Boas, E.R. Silva, E. Sousa, J.R. Almeida, M. Pinto, M. Correia-da-Silva, and V. Vasconcelos.
Figures



Similar articles
-
Proof of Concept of Natural and Synthetic Antifouling Agents in Coatings.Mar Drugs. 2024 Jun 24;22(7):291. doi: 10.3390/md22070291. Mar Drugs. 2024. PMID: 39057400 Free PMC article. Review.
-
Gallic acid derivatives as inhibitors of mussel (Mytilus galloprovincialis) larval settlement: Lead optimization, biological evaluation and use in antifouling coatings.Bioorg Chem. 2022 Sep;126:105911. doi: 10.1016/j.bioorg.2022.105911. Epub 2022 May 25. Bioorg Chem. 2022. PMID: 35661617
-
New Cyclam-Based Fe(III) Complexes Coatings Targeting Cobetia marina Biofilms.Molecules. 2025 Feb 16;30(4):917. doi: 10.3390/molecules30040917. Molecules. 2025. PMID: 40005227 Free PMC article.
-
Eco-friendly non-biocide-release coatings for marine biofouling prevention.Sci Total Environ. 2019 Feb 10;650(Pt 2):2499-2511. doi: 10.1016/j.scitotenv.2018.10.010. Epub 2018 Oct 2. Sci Total Environ. 2019. PMID: 30293004
-
The Role of Natural and Synthetic Flavonoids in the Prevention of Marine Biofouling.Mar Drugs. 2024 Feb 2;22(2):77. doi: 10.3390/md22020077. Mar Drugs. 2024. PMID: 38393048 Free PMC article. Review.
Cited by
-
Failure Severity Prediction for Protective-Coating Disbondment via the Classification of Acoustic Emission Signals.Sensors (Basel). 2023 Jul 31;23(15):6833. doi: 10.3390/s23156833. Sensors (Basel). 2023. PMID: 37571616 Free PMC article.
-
A Chemical Toolbox to Unveil Synthetic Nature-Inspired Antifouling (NIAF) Compounds.Mar Drugs. 2024 Sep 12;22(9):416. doi: 10.3390/md22090416. Mar Drugs. 2024. PMID: 39330297 Free PMC article. Review.
-
Development of Antifouling Strategies for Marine Applications.Microorganisms. 2023 Jun 13;11(6):1568. doi: 10.3390/microorganisms11061568. Microorganisms. 2023. PMID: 37375070 Free PMC article. Review.
-
Derivatives of Trimethoxybenzoic Acid and Gallic Acid as Potential Efflux Pump Inhibitors: In Silico and In Vitro Studies.Int J Mol Sci. 2022 Nov 21;23(22):14468. doi: 10.3390/ijms232214468. Int J Mol Sci. 2022. PMID: 36430942 Free PMC article.
-
Proof of Concept of Natural and Synthetic Antifouling Agents in Coatings.Mar Drugs. 2024 Jun 24;22(7):291. doi: 10.3390/md22070291. Mar Drugs. 2024. PMID: 39057400 Free PMC article. Review.
References
-
- Callow M., Callow J.E. Marine biofouling: A sticky problem. Biologist. 2002;49:10–14. - PubMed
-
- Bressy C., Lejars M. Marine fouling: An overview. J. Ocean Technol. 2014;9:19–28.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

