Novel Labdane Diterpenes-Based Synthetic Derivatives: Identification of a Bifunctional Vasodilator That Inhibits CaV1.2 and Stimulates KCa1.1 Channels
- PMID: 36005518
- PMCID: PMC9410420
- DOI: 10.3390/md20080515
Novel Labdane Diterpenes-Based Synthetic Derivatives: Identification of a Bifunctional Vasodilator That Inhibits CaV1.2 and Stimulates KCa1.1 Channels
Abstract
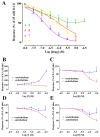
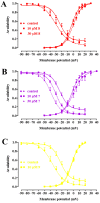
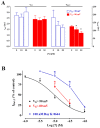
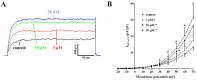
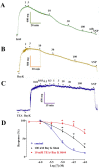
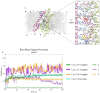
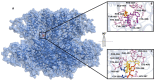
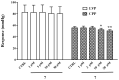
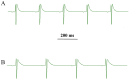
Sesquiterpenes such as leucodin and the labdane-type diterpene manool are natural compounds endowed with remarkably in vitro vasorelaxant and in vivo hypotensive activities. Given their structural similarity with the sesquiterpene lactone (+)-sclareolide, this molecule was selected as a scaffold to develop novel vasoactive agents. Functional, electrophysiology, and molecular dynamics studies were performed. The opening of the five-member lactone ring in the (+)-sclareolide provided a series of labdane-based small molecules, promoting a significant in vitro vasorelaxant effect. Electrophysiology data identified 7 as a CaV1.2 channel blocker and a KCa1.1 channel stimulator. These activities were also confirmed in the intact vascular tissue. The significant antagonism caused by the CaV1.2 channel agonist Bay K 8644 suggested that 7 might interact with the dihydropyridine binding site. Docking and molecular dynamic simulations provided the molecular basis of the CaV1.2 channel blockade and KCa1.1 channel stimulation produced by 7. Finally, 7 reduced coronary perfusion pressure and heart rate, while prolonging conduction and refractoriness of the atrioventricular node, likely because of its Ca2+ antagonism. Taken together, these data indicate that the labdane scaffold represents a valuable starting point for the development of new vasorelaxant agents endowed with negative chronotropic properties and targeting key pathways involved in the pathophysiology of hypertension and ischemic cardiomyopathy.
Keywords: CaV1.2 channels; KCa1.1 channels; Langendorff perfused heart; docking simulations; hypertension; labdane; molecular dynamics simulations; sclareolide; vasorelaxant activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A multitarget semi-synthetic derivative of the flavonoid morin with improved in vitro vasorelaxant activity: Role of CaV1.2 and KCa1.1 channels.Biochem Pharmacol. 2021 Mar;185:114429. doi: 10.1016/j.bcp.2021.114429. Epub 2021 Jan 26. Biochem Pharmacol. 2021. PMID: 33513341
-
Exploring the chemical space around chrysin to develop novel vascular CaV1.2 channel blockers, promising vasorelaxant agents.Arch Pharm (Weinheim). 2024 Nov;357(11):e2400536. doi: 10.1002/ardp.202400536. Epub 2024 Sep 6. Arch Pharm (Weinheim). 2024. PMID: 39239992
-
Artificial intelligence-driven identification of morin analogues acting as CaV1.2 channel blockers: Synthesis and biological evaluation.Bioorg Chem. 2023 Feb;131:106326. doi: 10.1016/j.bioorg.2022.106326. Epub 2022 Dec 17. Bioorg Chem. 2023. PMID: 36563413
-
Diterpene lactones with labdane, halimane and clerodane frameworks.Nat Prod Commun. 2011 Apr;6(4):497-504. Nat Prod Commun. 2011. PMID: 21560762 Review.
-
L-type CaV1.2 calcium channels: from in vitro findings to in vivo function.Physiol Rev. 2014 Jan;94(1):303-26. doi: 10.1152/physrev.00016.2013. Physiol Rev. 2014. PMID: 24382889 Review.
Cited by
-
Bioguided Identification of Polymethoxyflavones as Novel Vascular CaV1.2 Channel Blockers from Citrus Peel.Molecules. 2024 Dec 2;29(23):5693. doi: 10.3390/molecules29235693. Molecules. 2024. PMID: 39683852 Free PMC article.
-
Spirotetrahydroisoquinoline-Based Histone Deacetylase Inhibitors as New Antifibrotic Agents: Biological Evaluation in Human Fibroblasts from Bronchoalveolar Lavages of Idiopathic Pulmonary Fibrosis Patients.ACS Pharmacol Transl Sci. 2024 Nov 12;8(2):380-393. doi: 10.1021/acsptsci.4c00456. eCollection 2025 Feb 14. ACS Pharmacol Transl Sci. 2024. PMID: 39974640
-
A Comprehensive In Vitro and In Silico Approach for Targeting 4-Hydroxyphenyl Pyruvate Dioxygenase: Towards New Therapeutics for Alkaptonuria.Int J Mol Sci. 2025 Mar 29;26(7):3181. doi: 10.3390/ijms26073181. Int J Mol Sci. 2025. PMID: 40243989 Free PMC article.
-
Bioactive Potential of Sweet Cherry (Prunus avium L.) Waste: Antioxidant and Anti-Inflammatory Properties for Sustainable Applications.Foods. 2025 Apr 26;14(9):1523. doi: 10.3390/foods14091523. Foods. 2025. PMID: 40361606 Free PMC article.
-
Synthesis and Biological Evaluation of Sclareolide-Indole Conjugates and Their Derivatives.Molecules. 2023 Feb 11;28(4):1737. doi: 10.3390/molecules28041737. Molecules. 2023. PMID: 36838727 Free PMC article.
References
-
- Carullo G., Mazzotta S., Koch A., Hartmann K.M., Friedrich O., Gilbert D.F., Vega-Holm M., Schneider-Stock R., Aiello F. New oleoyl hybrids of natural antioxidants: Synthesis and in vitro evaluation as inducers of apoptosis in colorectal cancer cells. Antioxidants. 2020;9:1077. doi: 10.3390/antiox9111077. - DOI - PMC - PubMed
-
- Carullo G., Sciubba F., Governa P., Mazzotta S., Frattaruolo L., Grillo G., Cappello A.R., Cravotto G., Di Cocco M.E., Aiello F. Mantonico and pecorello grape seed extracts: Chemical characterization and evaluation of in vitro wound-healing and anti-inflammatory activities. Pharmaceuticals. 2020;13:97. doi: 10.3390/ph13050097. - DOI - PMC - PubMed
-
- Mazzotta S., Governa P., Borgonetti V., Marcolongo P., Nanni C., Gamberucci A., Manetti F., Pessina F., Carullo G., Brizzi A., et al. Pinocembrin and its linolenoyl ester derivative induce wound healing activity in HaCaT cell line potentially involving a GPR120/FFA4 mediated pathway. Bioorg. Chem. 2021;108:104657. doi: 10.1016/j.bioorg.2021.104657. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

