Mucoadhesive Marine Polysaccharides
- PMID: 36005525
- PMCID: PMC9409912
- DOI: 10.3390/md20080522
Mucoadhesive Marine Polysaccharides
Abstract
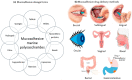
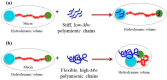
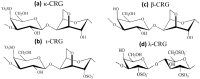
Mucoadhesive polymers are of growing interest in the field of drug delivery due to their ability to interact with the body's mucosa and increase the effectiveness of the drug. Excellent mucoadhesive performance is typically observed for polymers possessing charged groups or non-ionic functional groups capable of forming hydrogen bonds and electrostatic interactions with mucosal surfaces. Among mucoadhesive polymers, marine carbohydrate biopolymers have been attracting attention due to their biocompatibility and biodegradability, sample functional groups, strong water absorption and favorable physiochemical properties. Despite the large number of works devoted to mucoadhesive polymers, there are very few systematic studies on the influence of structural features of marine polysaccharides on mucoadhesive interactions. The purpose of this review is to characterize the mucoadhesive properties of marine carbohydrates with a focus on chitosan, carrageenan, alginate and their use in designing drug delivery systems. A wide variety of methods which have been used to characterize mucoadhesive properties of marine polysaccharides are presented in this review. Mucoadhesive drug delivery systems based on such polysaccharides are characterized by simplicity and ease of use in the form of tablets, gels and films through oral, buccal, transbuccal and local routes of administration.
Keywords: alginate; carrageenan; chitosan; methods of mucoadhesive; mucoadhesive; mucoadhesive interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery.Int J Biol Macromol. 2014 Mar;64:353-67. doi: 10.1016/j.ijbiomac.2013.12.017. Epub 2013 Dec 17. Int J Biol Macromol. 2014. PMID: 24360899 Review.
-
A Synthetic Bioinspired Carbohydrate Polymer with Mucoadhesive Properties.Angew Chem Int Ed Engl. 2020 Jan 7;59(2):704-710. doi: 10.1002/anie.201911720. Epub 2019 Nov 27. Angew Chem Int Ed Engl. 2020. PMID: 31701611 Free PMC article.
-
Current Status of Mucoadhesive Gel Systems for Buccal Drug Delivery.Curr Pharm Des. 2021;27(17):2015-2025. doi: 10.2174/1381612824666210316101528. Curr Pharm Des. 2021. PMID: 33726644 Review.
-
Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan--a review.J Control Release. 2006 Aug 10;114(1):1-14. doi: 10.1016/j.jconrel.2006.04.017. Epub 2006 May 22. J Control Release. 2006. PMID: 16828914 Review.
-
Advances in mucoadhesion and mucoadhesive polymers.Macromol Biosci. 2011 Jun 14;11(6):748-64. doi: 10.1002/mabi.201000388. Epub 2010 Dec 27. Macromol Biosci. 2011. PMID: 21188688 Review.
Cited by
-
Biopolymer Drug Delivery Systems for Oromucosal Application: Recent Trends in Pharmaceutical R&D.Int J Mol Sci. 2024 May 14;25(10):5359. doi: 10.3390/ijms25105359. Int J Mol Sci. 2024. PMID: 38791397 Free PMC article. Review.
-
Interaction of Liposomes Containing the Carrageenan/Echinochrome Complex with Human HaCaT Keratinocytes In Vitro.Mar Drugs. 2024 Dec 16;22(12):561. doi: 10.3390/md22120561. Mar Drugs. 2024. PMID: 39728136 Free PMC article.
-
Antiherpetic Activity of Carrageenan Complex with Echinochrome A and Its Liposomal Form.Int J Mol Sci. 2022 Dec 12;23(24):15754. doi: 10.3390/ijms232415754. Int J Mol Sci. 2022. PMID: 36555404 Free PMC article.
-
Effect of Chitosan on Rheological, Mechanical, and Adhesive Properties of Pectin-Calcium Gel.Mar Drugs. 2023 Jun 25;21(7):375. doi: 10.3390/md21070375. Mar Drugs. 2023. PMID: 37504906 Free PMC article.
-
Chitooligosaccharide enhanced the efficacy of Bacillus amyloliquefaciens CAS02 for the control of tobacco black shank.Front Microbiol. 2023 Nov 24;14:1296916. doi: 10.3389/fmicb.2023.1296916. eCollection 2023. Front Microbiol. 2023. PMID: 38075935 Free PMC article.
References
-
- Peppas N.A., Buri P.A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release. 1985;2:257–275. doi: 10.1016/0168-3659(85)90050-1. - DOI
-
- Kharenko E.A., Larionova N.I., Demina N.B. Mucoadhesive drug delivery systems (Review) Pharm. Chem. J. 2009;43:200–208. doi: 10.1007/s11094-009-0271-6. - DOI
-
- Sosnik A., das Neves J., Sarmento B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014;39:2030–2075. doi: 10.1016/j.progpolymsci.2014.07.010. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

