Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells
- PMID: 36005547
- PMCID: PMC9454251
- DOI: 10.1021/acs.est.2c03980
Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells
Abstract
Plastic debris in the global biosphere is an increasing concern, and nanoplastic (NPs) toxicity in humans is far from being understood. Studies have indicated that NPs can affect mitochondria, but the underlying mechanisms remain unclear. The liver and lungs have important metabolic functions and are vulnerable to NP exposure. In this study, we investigated the effects of 80 nm NPs on mitochondrial functions and metabolic pathways in normal human hepatic (L02) cells and lung (BEAS-2B) cells. NP exposure did not induce mass cell death; however, transmission electron microscopy analysis showed that the NPs could enter the cells and cause mitochondrial damage, as evidenced by overproduction of mitochondrial reactive oxygen species, alterations in the mitochondrial membrane potential, and suppression of mitochondrial respiration. These alterations were observed at NP concentrations as low as 0.0125 mg/mL, which might be comparable to the environmental levels. Nontarget metabolomics confirmed that the most significantly impacted processes were mitochondrial-related. The metabolic function of L02 cells was more vulnerable to NP exposure than that of BEAS-2B cells, especially at low NP concentrations. This study identifies NP-induced mitochondrial dysfunction and metabolic toxicity pathways in target human cells, providing insight into the possibility of adverse outcomes in human health.
Keywords: cytotoxicity; electron transport chain; energy metabolism; mitochondria; plastic particles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
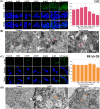

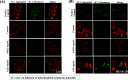

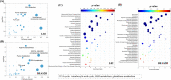

References
-
- Hernandez L. M.; Yousefi N.; Tufenkji N. Are there nanoplastics in your personal care products?. Environ. Sci. Technol. Lett. 2017, 4, 280–285. 10.1021/acs.estlett.7b00187. - DOI
-
- Lebreton L.; Andrady A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6.10.1057/s41599-018-0212-7. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

