Insight into Potential Interactions of Thyroid Hormones, Sex Hormones and Their Stimulating Hormones in the Development of Non-Alcoholic Fatty Liver Disease
- PMID: 36005590
- PMCID: PMC9414490
- DOI: 10.3390/metabo12080718
Insight into Potential Interactions of Thyroid Hormones, Sex Hormones and Their Stimulating Hormones in the Development of Non-Alcoholic Fatty Liver Disease
Abstract
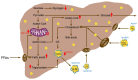
Non-Alcoholic Fatty Liver Disease (NAFLD) is a common manifestation of metabolic syndrome. In addition to lifestyle, endocrine hormones play a role in the dysregulation of hepatic metabolism. The most common endocrine hormones contributing to metabolic syndrome are alterations in the levels of thyroid hormones (THs, predominantly in subclinical hypothyroidism) and of sex hormones (in menopause). These hormonal changes influence hepatic lipid and glucose metabolism and may increase hepatic fat accumulation. This review compares the effects of sex hormones, THs and the respective stimulating hormones, Thyroid-Stimulating Hormone (TSH) and Follicle-Stimulating Hormone (FSH), on the development of hepatosteatosis. TSH and FSH may be more relevant to the dysregulation of hepatic metabolism than the peripheral hormones because metabolic changes were identified when only levels of the stimulating hormones were abnormal and the peripheral hormones were still in the reference range. Increased TSH and FSH levels appear to have additive effects on the development of NAFLD and to act independently from each other.
Keywords: follicle-stimulating hormone; hypothyroidism; menopause; metabolic dysfunction-associated fatty liver disease; metabolic syndrome; thyroid-stimulating hormone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Understanding the Relationship between Nonalcoholic Fatty Liver Disease and Thyroid Disease.Int J Mol Sci. 2023 Sep 27;24(19):14605. doi: 10.3390/ijms241914605. Int J Mol Sci. 2023. PMID: 37834051 Free PMC article. Review.
-
Effects of Thyroid Hormones on Lipid Metabolism Pathologies in Non-Alcoholic Fatty Liver Disease.Biomedicines. 2022 May 25;10(6):1232. doi: 10.3390/biomedicines10061232. Biomedicines. 2022. PMID: 35740254 Free PMC article. Review.
-
Association between Thyroid-Stimulating Hormone Levels and Non-Alcoholic Fatty Liver Disease Is Not Independent from Metabolic Syndrome Criteria.Eur Thyroid J. 2018 Nov;7(6):302-307. doi: 10.1159/000492324. Epub 2018 Aug 29. Eur Thyroid J. 2018. PMID: 30574460 Free PMC article.
-
Higher free triiodothyronine is associated with non-alcoholic fatty liver disease in euthyroid subjects: the Lifelines Cohort Study.Metabolism. 2017 Feb;67:62-71. doi: 10.1016/j.metabol.2016.11.002. Epub 2016 Nov 6. Metabolism. 2017. PMID: 28081779
-
Thyroid-stimulating hormone is an independent risk factor of non-alcoholic fatty liver disease.JGH Open. 2019 Oct 1;4(3):400-404. doi: 10.1002/jgh3.12264. eCollection 2020 Jun. JGH Open. 2019. PMID: 32514444 Free PMC article.
Cited by
-
The Influence of Subclinical Hypothyroidism on Endocrine and Metabolic Characteristics in Patients with Polycystic Ovary Syndrome.Int J Womens Health. 2025 Apr 10;17:1019-1026. doi: 10.2147/IJWH.S517997. eCollection 2025. Int J Womens Health. 2025. PMID: 40231192 Free PMC article.
-
Nuclear receptors in metabolic, inflammatory, and oncologic diseases: mechanisms, therapeutic advances, and future directions.Eur J Med Res. 2025 Sep 9;30(1):843. doi: 10.1186/s40001-025-03073-6. Eur J Med Res. 2025. PMID: 40926269 Free PMC article. Review.
-
Icariin Alleviates Nonalcoholic Fatty Liver Disease in Polycystic Ovary Syndrome by Improving Liver Fatty Acid Oxidation and Inhibiting Lipid Accumulation.Molecules. 2023 Jan 5;28(2):517. doi: 10.3390/molecules28020517. Molecules. 2023. PMID: 36677577 Free PMC article.
-
[Nonalcoholic fatty liver disease : Hepatic manifestations of metabolic syndrome].Inn Med (Heidelb). 2023 Apr;64(4):323-328. doi: 10.1007/s00108-022-01448-z. Epub 2022 Dec 29. Inn Med (Heidelb). 2023. PMID: 36580094 Review. German.
-
What Do Higher Alanine Aminotransferase Levels Mean in Premature Ovarian Insufficiency?Reprod Sci. 2024 Feb;31(2):469-479. doi: 10.1007/s43032-023-01303-y. Epub 2023 Sep 18. Reprod Sci. 2024. PMID: 37723330
References
-
- Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., Zelber-Sagi S., Wai-Sun Wong V., Dufour J.F., Schattenberg J.M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

