A Novel and Cross-Species Active Mammalian INDY (NaCT) Inhibitor Ameliorates Hepatic Steatosis in Mice with Diet-Induced Obesity
- PMID: 36005604
- PMCID: PMC9413491
- DOI: 10.3390/metabo12080732
A Novel and Cross-Species Active Mammalian INDY (NaCT) Inhibitor Ameliorates Hepatic Steatosis in Mice with Diet-Induced Obesity
Abstract
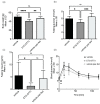
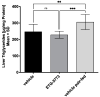
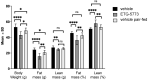
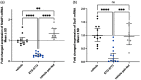
Mammalian INDY (mINDY, NaCT, gene symbol SLC13A5) is a potential target for the treatment of metabolically associated fatty liver disease (MAFLD). This study evaluated the effects of a selective, cross-species active, non-competitive, non-substrate-like inhibitor of NaCT. First, the small molecule inhibitor ETG-5773 was evaluated for citrate and succinate uptake and fatty acid synthesis in cell lines expressing both human NaCT and mouse Nact. Once its suitability was established, the inhibitor was evaluated in a diet-induced obesity (DIO) mouse model. DIO mice treated with 15 mg/kg compound ETG-5773 twice daily for 28 days had reduced body weight, fasting blood glucose, and insulin, and improved glucose tolerance. Liver triglycerides were significantly reduced, and body composition was improved by reducing fat mass, supported by a significant reduction in the expression of genes for lipogenesis such as SREBF1 and SCD1. Most of these effects were also evident after a seven-day treatment with the same dose. Further mechanistic investigation in the seven-day study showed increased plasma β-hydroxybutyrate and activated hepatic adenosine monophosphate-activated protein kinase (AMPK), reflecting findings from Indy (-/-) knockout mice. These results suggest that the inhibitor ETG-5773 blocked citrate uptake mediated by mouse and human NaCT to reduce liver steatosis and body fat and improve glucose regulation, proving the concept of NaCT inhibition as a future liver treatment for MAFLD.
Keywords: MAFLD; NAFLD; NASH; NaCT; SLC13A5; citrate transporter; mINDY; metabolic dysfunction.
Conflict of interest statement
G.Z. is an employee and shareholder of Eternygen GmbH; A.L.B. and J.J. are founders and shareholders of Eternygen GmbH. All other authors declare no conflict of interest. C.Y., R.J.-P., K.S., S.S., A.K.C.W., T.Z., and W.G. are employed by Evotec (UK) Ltd., Evotec SE, Evotec International GmbH, Aptuit (Verona) Srl, an Evotec Company, and their research and authorship of this article were completed within the scope of their employment.
Figures



Similar articles
-
Inhibition of citrate cotransporter Slc13a5/mINDY by RNAi improves hepatic insulin sensitivity and prevents diet-induced non-alcoholic fatty liver disease in mice.Mol Metab. 2016 Aug 13;5(11):1072-1082. doi: 10.1016/j.molmet.2016.08.004. eCollection 2016 Nov. Mol Metab. 2016. PMID: 27818933 Free PMC article.
-
The longevity gene INDY (I'm Not Dead Yet) in metabolic control: Potential as pharmacological target.Pharmacol Ther. 2018 May;185:1-11. doi: 10.1016/j.pharmthera.2017.10.003. Epub 2017 Oct 5. Pharmacol Ther. 2018. PMID: 28987323 Review.
-
Functional analysis of a species-specific inhibitor selective for human Na+-coupled citrate transporter (NaCT/SLC13A5/mINDY).Biochem J. 2020 Nov 13;477(21):4149-4165. doi: 10.1042/BCJ20200592. Biochem J. 2020. PMID: 33079129 Free PMC article.
-
Consequences of NaCT/SLC13A5/mINDY deficiency: good versus evil, separated only by the blood-brain barrier.Biochem J. 2021 Feb 12;478(3):463-486. doi: 10.1042/BCJ20200877. Biochem J. 2021. PMID: 33544126 Free PMC article. Review.
-
Arylhydrocarbon receptor-dependent mIndy (Slc13a5) induction as possible contributor to benzo[a]pyrene-induced lipid accumulation in hepatocytes.Toxicology. 2015 Nov 4;337:1-9. doi: 10.1016/j.tox.2015.08.007. Epub 2015 Aug 21. Toxicology. 2015. PMID: 26303333
Cited by
-
The impact of solute carrier proteins on disrupting substance regulation in metabolic disorders: insights and clinical applications.Front Pharmacol. 2025 Jan 9;15:1510080. doi: 10.3389/fphar.2024.1510080. eCollection 2024. Front Pharmacol. 2025. PMID: 39850557 Free PMC article. Review.
-
Membrane transporter progressive ankylosis protein homologue (ANKH/Ank) partially mediates senescence-derived extracellular citrate and is regulated by DNA damage, inflammation, and ageing.Front Aging. 2025 Jun 4;6:1583288. doi: 10.3389/fragi.2025.1583288. eCollection 2025. Front Aging. 2025. PMID: 40535023 Free PMC article.
-
Mapping the Metabolic Niche of Citrate Metabolism and SLC13A5.Metabolites. 2023 Feb 23;13(3):331. doi: 10.3390/metabo13030331. Metabolites. 2023. PMID: 36984771 Free PMC article. Review.
-
The Role of Solute Carrier Family Transporters in Hepatic Steatosis and Hepatic Fibrosis.J Clin Transl Hepatol. 2025 Mar 28;13(3):233-252. doi: 10.14218/JCTH.2024.00348. Epub 2025 Jan 22. J Clin Transl Hepatol. 2025. PMID: 40078199 Free PMC article. Review.
-
Targeting Longevity Gene SLC13A5: A Novel Approach to Prevent Age-Related Bone Fragility and Osteoporosis.Metabolites. 2023 Dec 6;13(12):1186. doi: 10.3390/metabo13121186. Metabolites. 2023. PMID: 38132868 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

