Nicotine Exposure during Rodent Pregnancy Alters the Composition of Maternal Gut Microbiota and Abundance of Maternal and Amniotic Short Chain Fatty Acids
- PMID: 36005607
- PMCID: PMC9414314
- DOI: 10.3390/metabo12080735
Nicotine Exposure during Rodent Pregnancy Alters the Composition of Maternal Gut Microbiota and Abundance of Maternal and Amniotic Short Chain Fatty Acids
Abstract
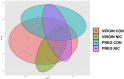
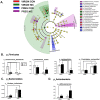
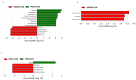
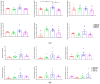
Tobacco smoking is the leading cause of preventable death. Numerous reports link smoking in pregnancy with serious adverse outcomes, such as miscarriage, stillbirth, prematurity, low birth weight, perinatal morbidity, and infant mortality. Corollaries of consuming nicotine in pregnancy, separate from smoking, are less explored, and the mechanisms of nicotine action on maternal-fetal communication are poorly understood. This study examined alterations in the maternal gut microbiome in response to nicotine exposure during pregnancy. We report that changes in the maternal gut microbiota milieu are an important intermediary that may mediate the prenatal nicotine exposure effects, affect gene expression, and alter fetal exposure to circulating short-chain fatty acids (SCFAs) and leptin during in utero development.
Keywords: SCFA; microbiota; pregnancy; smoking.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Electroacupuncture may protect pulmonary dysplasia in offspring with perinatal nicotine exposure by altering maternal gut microbiota and metabolites.Front Microbiol. 2025 Jan 9;15:1465673. doi: 10.3389/fmicb.2024.1465673. eCollection 2024. Front Microbiol. 2025. PMID: 39850138 Free PMC article.
-
Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations.J Physiol. 2018 Oct;596(20):4923-4944. doi: 10.1113/JP276431. Epub 2018 Aug 28. J Physiol. 2018. PMID: 30066368 Free PMC article.
-
Fetal nicotine or cocaine exposure: which one is worse?J Pharmacol Exp Ther. 1998 Jun;285(3):931-45. J Pharmacol Exp Ther. 1998. PMID: 9618392
-
Prenatal nicotine exposure during pregnancy results in adverse neurodevelopmental alterations and neurobehavioral deficits.Adv Drug Alcohol Res. 2023 Aug 11;3:11628. doi: 10.3389/adar.2023.11628. eCollection 2023. Adv Drug Alcohol Res. 2023. PMID: 38389806 Free PMC article. Review.
-
Perinatal nicotine/smoking exposure and carotid chemoreceptors during development.Respir Physiol Neurobiol. 2013 Jan 1;185(1):110-9. doi: 10.1016/j.resp.2012.06.023. Epub 2012 Jun 26. Respir Physiol Neurobiol. 2013. PMID: 22743051 Review.
Cited by
-
Early-life exposures and the microbiome: implications for IBD prevention.Gut. 2024 Feb 23;73(3):541-549. doi: 10.1136/gutjnl-2023-330002. Gut. 2024. PMID: 38123972 Free PMC article. Review.
-
Chronotropic and vasoactive properties of the gut bacterial short-chain fatty acids in larval zebrafish.Physiol Genomics. 2024 Jun 1;56(6):426-435. doi: 10.1152/physiolgenomics.00013.2024. Epub 2024 Apr 1. Physiol Genomics. 2024. PMID: 38557279 Free PMC article.
-
Intestinal flora and pregnancy complications: Current insights and future prospects.Imeta. 2024 Jan 22;3(2):e167. doi: 10.1002/imt2.167. eCollection 2024 Apr. Imeta. 2024. PMID: 38882493 Free PMC article. Review.
-
Shaping immunity: the influence of the maternal gut bacteria on fetal immune development.Semin Immunopathol. 2025 Feb 1;47(1):13. doi: 10.1007/s00281-025-01039-8. Semin Immunopathol. 2025. PMID: 39891756 Free PMC article. Review.
-
Electroacupuncture may protect pulmonary dysplasia in offspring with perinatal nicotine exposure by altering maternal gut microbiota and metabolites.Front Microbiol. 2025 Jan 9;15:1465673. doi: 10.3389/fmicb.2024.1465673. eCollection 2024. Front Microbiol. 2025. PMID: 39850138 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

