Impact of Maternal Obesity on the Gestational Metabolome and Infant Metabolome, Brain, and Behavioral Development in Rhesus Macaques
- PMID: 36005637
- PMCID: PMC9415340
- DOI: 10.3390/metabo12080764
Impact of Maternal Obesity on the Gestational Metabolome and Infant Metabolome, Brain, and Behavioral Development in Rhesus Macaques
Abstract
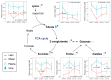
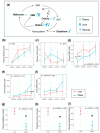
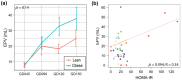
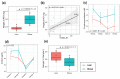
Maternal gestational obesity is associated with elevated risks for neurodevelopmental disorder, including autism spectrum disorder. However, the mechanisms by which maternal adiposity influences fetal developmental programming remain to be elucidated. We aimed to understand the impact of maternal obesity on the metabolism of both pregnant mothers and their offspring, as well as on metabolic, brain, and behavioral development of offspring by utilizing metabolomics, protein, and behavioral assays in a non-human primate model. We found that maternal obesity was associated with elevated inflammation and significant alterations in metabolites of energy metabolism and one-carbon metabolism in maternal plasma and urine, as well as in the placenta. Infants that were born to obese mothers were significantly larger at birth compared to those that were born to lean mothers. Additionally, they exhibited significantly reduced novelty preference and significant alterations in their emotional response to stress situations. These changes coincided with differences in the phosphorylation of enzymes in the brain mTOR signaling pathway between infants that were born to obese and lean mothers and correlated with the concentration of maternal plasma betaine during pregnancy. In summary, gestational obesity significantly impacted the infant systemic and brain metabolome and adaptive behaviors.
Keywords: NMR; behavior; brain; infant development; metabolomics; obesity; placenta; plasma; pregnancy; urine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Calorie restriction and pravastatin administration during pregnancy in obese rhesus macaques modulates maternal and infant metabolism and infant brain and behavioral development.Front Nutr. 2023 May 15;10:1146804. doi: 10.3389/fnut.2023.1146804. eCollection 2023. Front Nutr. 2023. PMID: 37255938 Free PMC article.
-
Association of polycystic ovary syndrome or anovulatory infertility with offspring psychiatric and mild neurodevelopmental disorders: a Finnish population-based cohort study.Hum Reprod. 2020 Oct 1;35(10):2336-2347. doi: 10.1093/humrep/deaa192. Hum Reprod. 2020. PMID: 32866965 Free PMC article.
-
Offspring body size and metabolic profile - effects of lifestyle intervention in obese pregnant women.Dan Med J. 2014 Jul;61(7):B4893. Dan Med J. 2014. PMID: 25123127 Review.
-
Transgenerational cycle of obesity and diabetes: investigating possible metabolic precursors in cord blood from the PREOBE study.Acta Diabetol. 2019 Sep;56(9):1073-1082. doi: 10.1007/s00592-019-01349-y. Epub 2019 May 6. Acta Diabetol. 2019. PMID: 31062097
-
Placental function in maternal obesity.Clin Sci (Lond). 2020 Apr 30;134(8):961-984. doi: 10.1042/CS20190266. Clin Sci (Lond). 2020. PMID: 32313958 Free PMC article. Review.
Cited by
-
The Autism Spectrum Disorder and Its Possible Origins in Pregnancy.Int J Environ Res Public Health. 2024 Feb 20;21(3):244. doi: 10.3390/ijerph21030244. Int J Environ Res Public Health. 2024. PMID: 38541246 Free PMC article. Review.
-
Calorie restriction and pravastatin administration during pregnancy in obese rhesus macaques modulates maternal and infant metabolism and infant brain and behavioral development.Front Nutr. 2023 May 15;10:1146804. doi: 10.3389/fnut.2023.1146804. eCollection 2023. Front Nutr. 2023. PMID: 37255938 Free PMC article.
-
Autism spectrum disorders and the gastrointestinal tract: insights into mechanisms and clinical relevance.Nat Rev Gastroenterol Hepatol. 2024 Mar;21(3):142-163. doi: 10.1038/s41575-023-00857-1. Epub 2023 Dec 19. Nat Rev Gastroenterol Hepatol. 2024. PMID: 38114585 Review.
References
-
- Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

