Similar 5F-APINACA Metabolism between CD-1 Mouse and Human Liver Microsomes Involves Different P450 Cytochromes
- PMID: 36005645
- PMCID: PMC9413144
- DOI: 10.3390/metabo12080773
Similar 5F-APINACA Metabolism between CD-1 Mouse and Human Liver Microsomes Involves Different P450 Cytochromes
Abstract
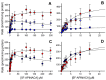
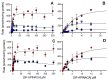
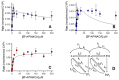
In 2019, synthetic cannabinoids accounted for more than one-third of new drugs of abuse worldwide; however, assessment of associated health risks is not ethical for controlled and often illegal substances, making CD-1 mouse exposure studies the gold standard. Interpretation of those findings then depends on the similarity of mouse and human metabolic pathways. Herein, we report the first comparative analysis of steady-state metabolism of N-(1-adamantyl)-1-(5-pentyl)-1H-indazole-3-carboxamide (5F-APINACA/5F-AKB48) in CD-1 mice and humans using hepatic microsomes. Regardless of species, 5F-APINACA metabolism involved highly efficient sequential adamantyl hydroxylation and oxidative defluorination pathways that competed equally. Secondary adamantyl hydroxylation was less efficient for mice. At low 5F-APINACA concentrations, initial rates were comparable between pathways, but at higher concentrations, adamantyl hydroxylations became less significant due to substrate inhibition likely involving an effector site. For humans, CYP3A4 dominated both metabolic pathways with minor contributions from CYP2C8, 2C19, and 2D6. For CD-1 mice, Cyp3a11 and Cyp2c37, Cyp2c50, and Cyp2c54 contributed equally to adamantyl hydroxylation, but Cyp3a11 was more efficient at oxidative defluorination than Cyp2c members. Taken together, the results of our in vitro steady-state study indicate a high conservation of 5F-APINACA metabolism between CD-1 mice and humans, but deviations can occur due to differences in P450s responsible for the associated reactions.
Keywords: 5F-AKB48; 5F-APINACA; CB1 receptor; P450; drug abuse; enzyme kinetics; human; metabolism; mouse; synthetic cannabinoid.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Significance of Competing Metabolic Pathways for 5F-APINACA Based on Quantitative Kinetics.Molecules. 2020 Oct 20;25(20):4820. doi: 10.3390/molecules25204820. Molecules. 2020. PMID: 33092129 Free PMC article.
-
In Vitro Inhibitory Effects of APINACA on Human Major Cytochrome P450, UDP-Glucuronosyltransferase Enzymes, and Drug Transporters.Molecules. 2019 Aug 19;24(16):3000. doi: 10.3390/molecules24163000. Molecules. 2019. PMID: 31430908 Free PMC article.
-
In Vitro Phase I Metabolic Profiling of the Synthetic Cannabinoids AM-694, 5F-NNEI, FUB-APINACA, MFUBINAC, and AMB-FUBINACA.Chem Res Toxicol. 2020 Jul 20;33(7):1653-1664. doi: 10.1021/acs.chemrestox.9b00466. Epub 2020 Apr 28. Chem Res Toxicol. 2020. PMID: 32301604
-
Isomeric discrimination of synthetic cannabinoids by GC-EI-MS: 1-adamantyl and 2-adamantyl isomers of N-adamantyl carboxamides.Drug Test Anal. 2017 Mar;9(3):378-388. doi: 10.1002/dta.2124. Epub 2016 Nov 18. Drug Test Anal. 2017. PMID: 27770510
-
Identification of three cannabimimetic indazole and pyrazole derivatives, APINACA 2H-indazole analogue, AMPPPCA, and 5F-AMPPPCA.Drug Test Anal. 2017 Feb;9(2):248-255. doi: 10.1002/dta.1967. Epub 2016 Mar 9. Drug Test Anal. 2017. PMID: 26968728
Cited by
-
Synthetic cannabinoid receptor agonists exacerbate fentanyl-elicited respiratory depression and confer resistance to naloxone rescue in mice.Drug Alcohol Depend. 2025 Jul 1;272:112672. doi: 10.1016/j.drugalcdep.2025.112672. Epub 2025 Apr 15. Drug Alcohol Depend. 2025. PMID: 40319790
References
-
- News: January 2020—UNODC-SMART: Share of NPS Stimulants and Synthetic Cannabinoids Large but Stable While Opioids Increase. [(accessed on 22 September 2020)]. Available online: https://www.unodc.org/LSS/Announcement/Details/0096a6ec-c5d0-404f-a559-d....
-
- Van Hout M.C., Hearne E. User Experiences of Development of Dependence on the Synthetic Cannabinoids, 5f-AKB48 and 5F-PB-22, and Subsequent Withdrawal Syndromes. Int. J. Ment. Health Addict. 2017;15:565–579. doi: 10.1007/s11469-016-9650-x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

