Evaluation of the Growth-Inhibitory Spectrum of Three Types of Cyanoacrylate Nanoparticles on Gram-Positive and Gram-Negative Bacteria
- PMID: 36005697
- PMCID: PMC9414559
- DOI: 10.3390/membranes12080782
Evaluation of the Growth-Inhibitory Spectrum of Three Types of Cyanoacrylate Nanoparticles on Gram-Positive and Gram-Negative Bacteria
Abstract
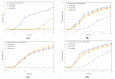
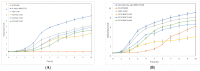
The development of novel effective antibacterial agents is crucial due to increasing antibiotic resistance in various bacteria. Poly (alkyl cyanoacrylate) nanoparticles (PACA-NPs) are promising novel antibacterial agents as they have shown antibacterial activity against several Gram-positive and Gram-negative bacteria. However, the antibacterial mechanism remains unclear. Here, we compared the antibacterial efficacy of ethyl cyanoacrylate nanoparticles (ECA-NPs), isobutyl cyanoacrylate NPs (iBCA-NPs), and ethoxyethyl cyanoacrylate NPs (EECA-NPs) using five Gram-positive and five Gram-negative bacteria. Among these resin nanoparticles, ECA-NPs showed the highest growth inhibitory effect against all the examined bacterial species, and this effect was higher against Gram-positive bacteria than Gram-negative. While iBCA-NP could inhibit the cell growth only in two Gram-positive bacteria, i.e., Bacillus subtilis and Staphylococcus aureus, it had negligible inhibitory effect against all five Gram-negative bacteria examined. Irrespective of the differences in growth inhibition induced by these three NPs, N-acetyl-L-cysteine (NAC), a well-known reactive oxygen species (ROS) scavenger, efficiently restored growth in all the bacterial strains to that similar to untreated cells. This strongly suggests that the exposure to NPs generates ROS, which mainly induces cell growth inhibition irrespective of the difference in bacterial species and cyanoacrylate NPs used.
Keywords: antibacterial agent; cyanoacrylate nanoparticle; membrane damage; reactive oxygen species; stress response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
In Vitro Efficacy of Isobutyl Cyanoacrylate Nanoparticles against Fish Bacterial Pathogens and Selection Preference by Rainbow Trout (Oncorhynchus mykiss).Microorganisms. 2023 Nov 28;11(12):2877. doi: 10.3390/microorganisms11122877. Microorganisms. 2023. PMID: 38138020 Free PMC article.
-
The Molecular Mechanisms of the Antibacterial Effect of Picosecond Laser Generated Silver Nanoparticles and Their Toxicity to Human Cells.PLoS One. 2016 Aug 30;11(8):e0160078. doi: 10.1371/journal.pone.0160078. eCollection 2016. PLoS One. 2016. PMID: 27575485 Free PMC article.
-
A novel study of antibacterial activity of copper iodide nanoparticle mediated by DNA and membrane damage.Colloids Surf B Biointerfaces. 2012 Aug 1;96:50-5. doi: 10.1016/j.colsurfb.2012.03.021. Epub 2012 Apr 5. Colloids Surf B Biointerfaces. 2012. PMID: 22521682
-
Antibacterial activities of transient metals nanoparticles and membranous mechanisms of action.World J Microbiol Biotechnol. 2019 Oct 14;35(10):162. doi: 10.1007/s11274-019-2742-6. World J Microbiol Biotechnol. 2019. PMID: 31612285 Review.
-
Transition metal-based nanoparticles as potential antimicrobial agents: recent advancements, mechanistic, challenges, and future prospects.Discov Nano. 2023 Jun 7;18(1):84. doi: 10.1186/s11671-023-03861-1. Discov Nano. 2023. PMID: 37382784 Free PMC article. Review.
Cited by
-
In Vitro Efficacy of Isobutyl Cyanoacrylate Nanoparticles against Fish Bacterial Pathogens and Selection Preference by Rainbow Trout (Oncorhynchus mykiss).Microorganisms. 2023 Nov 28;11(12):2877. doi: 10.3390/microorganisms11122877. Microorganisms. 2023. PMID: 38138020 Free PMC article.
References
-
- Munita J.M., Arias C.A. Virulence Mechanisms of Bacterial Pathogens. John Wiley and Sons; Hoboken, NJ, USA: 2016. Mechanisms of Antibiotic Resistance; pp. 481–511.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

