Population Pharmacokinetic Meta-Analysis and Dosing Recommendation for Meropenem in Critically Ill Patients Receiving Continuous Renal Replacement Therapy
- PMID: 36005753
- PMCID: PMC9487629
- DOI: 10.1128/aac.00822-22
Population Pharmacokinetic Meta-Analysis and Dosing Recommendation for Meropenem in Critically Ill Patients Receiving Continuous Renal Replacement Therapy
Abstract
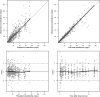
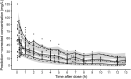
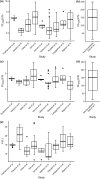
The optimal dosing regimen for meropenem in critically ill patients undergoing continuous renal replacement therapy (CRRT) remains undefined due to small studied sample sizes and uninformative pharmacokinetic (PK)/pharmacodynamic (PD) analyses in reported studies. The present study aimed to perform a population PK/PD meta-analysis of meropenem using available literature data to suggest the optimal treatment regimen. A total of 501 meropenem concentration measurements from 78 adult CRRT patients pooled from nine published studies were used to develop the population PK model for meropenem. PK/PD target (40% and 100% of the time with the unbound drug plasma concentration above the MIC) marker-based efficacy and risk of toxicity (trough concentrations of >45 mg/L) for short-term (30 min), prolonged (3 h), and continuous (24 h) infusion dosing strategies for meropenem were investigated. The impact of CRRT dose and identified covariates on the PD probability of target attainment (PTA) and predicted toxicity was also examined. Meropenem concentration data were adequately described by a two-compartment model with linear elimination. Trauma was identified as a pronounced modifier for endogenous clearance of meropenem. Simulations demonstrated that adequate PK/PD targets and low risk of toxicity could be achieved in non-trauma CRRT patients receiving meropenem regimens of 1 g every 6 h infused over 30 min, 1 g every 8 h infused over 3 h, and 2 to 4 g every 24 h infused over 24 h. The impact of CRRT dose (25 to 50 mL/kg/h) on PTA was clinically irrelevant, and continuous infusion of 3 to 4 g every 24 h was suitable for trauma CRRT patients (MICs of ≤0.5 mg/L). A population PK model was developed for meropenem in CRRT patients, and different dosing regimens were proposed for non-trauma and trauma CRRT patients.
Keywords: NONMEM; continuous renal replacement therapy; critically ill; meropenem; population pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Population Pharmacokinetics of Prolonged Infusion for Meropenem: Tailoring Dosing Recommendations for Chinese Critically Ill Patients on Continuous Renal Replacement Therapy with Consideration for Renal Function.Drug Des Devel Ther. 2025 Feb 17;19:1105-1117. doi: 10.2147/DDDT.S489603. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 39991086 Free PMC article.
-
Optimal Meropenem Dosing Regimens in Patients Undergoing Continuous Renal Replacement Therapy: Systematic Review and Monte Carlo Simulations.Blood Purif. 2023;52(6):503-515. doi: 10.1159/000529694. Epub 2023 May 5. Blood Purif. 2023. PMID: 37231811
-
Population pharmacokinetics of standard-dose meropenem in critically ill patients on continuous renal replacement therapy: a prospective observational trial.Pharmacol Rep. 2020 Jun;72(3):719-729. doi: 10.1007/s43440-020-00104-3. Epub 2020 Apr 16. Pharmacol Rep. 2020. PMID: 32301057 Free PMC article.
-
Population Pharmacokinetics and Dosing Optimization of Gentamicin in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy.Drug Des Devel Ther. 2022 Jan 6;16:13-22. doi: 10.2147/DDDT.S343385. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 35023902 Free PMC article.
-
Pharmacokinetics of piperacillin and tazobactam in critically Ill patients treated with continuous kidney replacement therapy: A mini-review and population pharmacokinetic analysis.J Clin Pharm Ther. 2022 Aug;47(8):1091-1102. doi: 10.1111/jcpt.13657. Epub 2022 Mar 29. J Clin Pharm Ther. 2022. PMID: 35352374 Free PMC article. Review.
Cited by
-
Covariates in population pharmacokinetic studies of critically ill adults receiving β-lactam antimicrobials: a systematic review and narrative synthesis.JAC Antimicrob Resist. 2024 Feb 26;6(1):dlae030. doi: 10.1093/jacamr/dlae030. eCollection 2024 Feb. JAC Antimicrob Resist. 2024. PMID: 38410250 Free PMC article. Review.
-
Global research on the utilization of population pharmacokinetic model: a bibliometric analysis from 2000 to 2024.Front Pharmacol. 2025 May 12;16:1548023. doi: 10.3389/fphar.2025.1548023. eCollection 2025. Front Pharmacol. 2025. PMID: 40421221 Free PMC article. Review.
-
How to use meropenem in pediatric patients undergoing CKRT? Integrated meropenem pharmacokinetic model for critically ill children.Antimicrob Agents Chemother. 2024 Jun 5;68(6):e0172923. doi: 10.1128/aac.01729-23. Epub 2024 Apr 24. Antimicrob Agents Chemother. 2024. PMID: 38656186 Free PMC article.
-
Kidney replacement therapy during extracorporeal membrane oxygenation: pathophysiology, technical considerations, and outcomes.Ren Fail. 2025 Dec;47(1):2486557. doi: 10.1080/0886022X.2025.2486557. Epub 2025 Apr 23. Ren Fail. 2025. PMID: 40265202 Free PMC article. Review.
-
Meropenem Pharmacokinetics and Target Attainment in Critically Ill Patients.Infect Drug Resist. 2023 Jun 21;16:3989-3997. doi: 10.2147/IDR.S408572. eCollection 2023. Infect Drug Resist. 2023. PMID: 37366501 Free PMC article.
References
-
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. 2016. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315:801–810. doi:10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Rizk ML, Bhavnani SM, Drusano G, Dane A, Eakin AE, Guina T, Jang SH, Tomayko JF, Wang J, Zhuang L, Lodise TP. 2019. Considerations for dose selection and clinical pharmacokinetics/pharmacodynamics for the development of antibacterial agents. Antimicrob Agents Chemother 63:e02309-18. doi:10.1128/AAC.02309-18. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

