Activity of Tricyclic Pyrrolopyrimidine Gyrase B Inhibitor against Mycobacterium abscessus
- PMID: 36005813
- PMCID: PMC9487482
- DOI: 10.1128/aac.00669-22
Activity of Tricyclic Pyrrolopyrimidine Gyrase B Inhibitor against Mycobacterium abscessus
Abstract
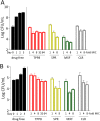
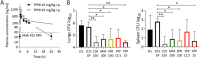
Tricyclic pyrrolopyrimidines (TPPs) are a new class of antibacterials inhibiting the ATPase of DNA gyrase. TPP8, a representative of this class, is active against Mycobacterium abscessus in vitro. Spontaneous TPP8 resistance mutations mapped to the ATPase domain of M. abscessus DNA gyrase, and the compound inhibited DNA supercoiling activity of recombinant M. abscessus enzyme. Further profiling of TPP8 in macrophage and mouse infection studies demonstrated proof-of-concept activity against M. abscessus ex vivo and in vivo.
Keywords: DNA gyrase; NTM; SPR719; nontuberculous mycobacteria.
Conflict of interest statement
The authors declare a conflict of interest. A.E.M., R.R.M., C.W.B., N.M., C.J.B., and D.B.O. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA.
Figures

Similar articles
-
In Vitro Resistance against DNA Gyrase Inhibitor SPR719 in Mycobacterium avium and Mycobacterium abscessus.Microbiol Spectr. 2022 Feb 23;10(1):e0132121. doi: 10.1128/spectrum.01321-21. Epub 2022 Jan 12. Microbiol Spectr. 2022. PMID: 35019671 Free PMC article.
-
Piperidine-4-Carboxamides Target DNA Gyrase in Mycobacterium abscessus.Antimicrob Agents Chemother. 2021 Jul 16;65(8):e0067621. doi: 10.1128/AAC.00676-21. Epub 2021 Jul 16. Antimicrob Agents Chemother. 2021. PMID: 34001512 Free PMC article.
-
A Mycobacterium tuberculosis NBTI DNA Gyrase Inhibitor Is Active against Mycobacterium abscessus.Antimicrob Agents Chemother. 2021 Nov 17;65(12):e0151421. doi: 10.1128/AAC.01514-21. Epub 2021 Oct 4. Antimicrob Agents Chemother. 2021. PMID: 34606340 Free PMC article.
-
Omadacycline for management of Mycobacterium abscessus infections: a review of its effectiveness, place in therapy, and considerations for use.BMC Infect Dis. 2022 Nov 22;22(1):874. doi: 10.1186/s12879-022-07857-7. BMC Infect Dis. 2022. PMID: 36419143 Free PMC article. Review.
-
A review of current and promising nontuberculous mycobacteria antibiotics.Future Med Chem. 2021 Aug;13(16):1367-1395. doi: 10.4155/fmc-2021-0048. Epub 2021 Jun 24. Future Med Chem. 2021. PMID: 34165325 Review.
Cited by
-
Toward a Bactericidal Oral Drug Combination for the Treatment of Mycobacterium abscessus Lung Disease.ACS Infect Dis. 2025 Apr 11;11(4):929-939. doi: 10.1021/acsinfecdis.4c00948. Epub 2025 Apr 1. ACS Infect Dis. 2025. PMID: 40168319 Free PMC article.
-
DS86760016, a Leucyl-tRNA Synthetase Inhibitor, Is Active against Mycobacterium abscessus.Antimicrob Agents Chemother. 2023 Jun 15;67(6):e0156722. doi: 10.1128/aac.01567-22. Epub 2023 May 22. Antimicrob Agents Chemother. 2023. PMID: 37212672 Free PMC article. Clinical Trial.
-
Durlobactam to boost the clinical utility of standard of care β-lactams against Mycobacterium abscessus lung disease.Antimicrob Agents Chemother. 2025 Jan 31;69(1):e0104624. doi: 10.1128/aac.01046-24. Epub 2024 Nov 20. Antimicrob Agents Chemother. 2025. PMID: 39565116 Free PMC article.
-
Why Matter Matters: Fast-Tracking Mycobacterium abscessus Drug Discovery.Molecules. 2022 Oct 17;27(20):6948. doi: 10.3390/molecules27206948. Molecules. 2022. PMID: 36296540 Free PMC article. Review.
-
Preclinical murine models for the testing of antimicrobials against Mycobacterium abscessus pulmonary infections: Current practices and recommendations.Tuberculosis (Edinb). 2024 Jul;147:102503. doi: 10.1016/j.tube.2024.102503. Epub 2024 Mar 19. Tuberculosis (Edinb). 2024. PMID: 38729070 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

