Oxy-Borylenes as Photoreductants: Synthesis and Application in Dehalogenation and Detosylation Reactions
- PMID: 36005897
- PMCID: PMC9825981
- DOI: 10.1002/anie.202209391
Oxy-Borylenes as Photoreductants: Synthesis and Application in Dehalogenation and Detosylation Reactions
Abstract
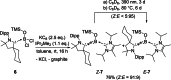
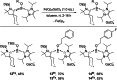
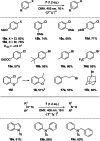
While the range of accessible borylenes has significantly broadened over the last decade, applications remain limited. Herein, we present tricoordinate oxy-borylenes as potent photoreductants that can be readily activated by visible light. Facile oxidation of CAAC stabilized oxy-borylenes (CAAC)(IPr2 Me2 )BOR (R=TMS, CH2 CH2 C6 H5 , CH2 CH2 (4-F)C6 H4 ) to their corresponding radical cations is achieved with mildly oxidizing ferrocenium ion. Cyclovoltammetric studies reveal ground-state redox potentials of up to -1.90 V vs. Fc+/0 for such oxy-borylenes placing them among the strongest organic super electron donors. Their ability as photoreductants is further supported by theoretical studies and showcased by the application as stoichiometric reagents for the photochemical hydrodehalogenation of aryl chlorides, aryl bromides and unactivated alkyl bromides as well as the detosylation of anilines.
Keywords: Boron Compounds; EPR Spectroscopy; Photochemistry; Radical Reactions; SET Reduction.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


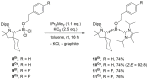


References
-
- Soleilhavoup M., Bertrand G., Angew. Chem. Int. Ed. 2017, 56, 10282–10292; - PubMed
- Angew. Chem. 2017, 129, 10416–10426.
-
- selected spectroscopic studies:
-
- Timms P. L., J. Am. Chem. Soc. 1967, 89, 1629–1632;
-
- Timms P. L., Acc. Chem. Res. 1973, 6, 118–123;
-
- Nomoto M., Okabayashi T., Klaus T., Tanimoto M., J. Mol. Struct. 1997, 413–414, 471–476;
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

