A prospective real-world study of the diffuse-type tenosynovial giant cell tumor patient journey: A 2-year observational analysis
- PMID: 36006054
- PMCID: PMC9804718
- DOI: 10.1002/jso.27067
A prospective real-world study of the diffuse-type tenosynovial giant cell tumor patient journey: A 2-year observational analysis
Abstract
Background and objectives: Diffuse-tenosynovial giant cell tumor (D-TGCT) is a rare, locally aggressive, typically benign neoplasm affecting mainly large joints, representing a wide clinical spectrum. We provide a picture of the treatment journey of D-TGCT patients as a 2-year observational follow-up.
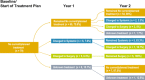
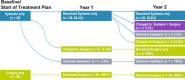
Methods: The TGCT Observational Platform Project registry was a multinational, multicenter, prospective observational study at tertiary sarcoma centers spanning seven European countries and two US sites. Histologically confirmed D-TGCT patients were categorized as either those who remained on initial treatment strategy (determined at baseline visit) or those who changed treatment strategy with specific changes documented (e.g., systemic treatment to surgery) at the 1-year and/or 2-year follow-up visits.
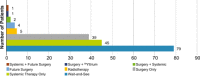
Results: A total of 176 patients were assessed, mean diagnosis age was 38.4 (SD ± 14.6) years; most patients had a knee tumor (120/176, 68.2%). For the 2-year observation period, most patients (75.5%) remained on the baseline treatment strategy throughout, 54/79 patients (68.4%) remained no treatment, 30/45 patients (66.7%) remained systemic treatment, 39/39 patients (100%) remained surgery. Those who changed treatment strategy utilized multimodal treatment options.
Conclusions: This is the first prospectively collected analysis to describe D-TGCT patient treatments over an extended follow-up and demonstrates the need for multidisciplinary teams to determine an optimal treatment strategy.
Keywords: TGCT observational platform project (TOPP); diffuse-tenosynovial giant cell tumor (D-TGCT); pexidartinib; prospective; real-world.
© 2022 The Authors. Journal of Surgical Oncology published by Wiley Periodicals LLC.
Conflict of interest statement
NMB reports consulting fees from Daiichi Sankyo, Zimmer Biomet, and Onkos Surgical. JHH reports consulting fees from Daiichi Sankyo and Stryker; payment for expert testimony from Martin, Magnuson, McCarthy & Kenney; leadership or fiduciary role in Clinical Orthopaedics and Related Research, Musculoskeletal Transplant Foundation, and MIB Agents (osteosarcoma). EP reports consulting fees for advisory board from Amgen, Daiichi Sankyo, Lilly, Eusa Pharma, Deciphera Pharmaceuticals; drugs for preclinical research from Bristol‐Myers Squibb, Pfizer, PharmaMar; medical writing support for preclinical research from PharmaMar; and support for attending meetings and/or travel from Lilly, PharmaMar, and Takeda. SB reports research funding from Incyte to institution; consulting fees from Adcendo, Bayer, Blueprint Medicine, Boehringer Ingelheim, Cogent, Daiichi Sankyo, Deciphera, Exelixis, GSK, Novartis; honoraria from BluePrint Medicine and Deciphera; unpaid participation on Data Safety Monitoring Board or Advisory Board for Novartis, and unpaid leadership or fiduciary role in Deutsche Sarkomstiftung. HS declares no competing interests. AL reports institution education grants from Johnson and Johnson, Alphamed, Medacta and Implantec. JMB reports grants or contracts from, honoraria from, and participation on a Data Safety Monitoring Board or Advisory board for Amgen, Asofarma, Bayer, GSK, Lilly, Novartis, PharmaMar, Roche, Tecnofarma; consulting fees, payment for expert testimony, and support for attending meetings and/or travel from PharmaMar. FG reports consulting fees from Amgen, stock ownership in and co‐founder of Atlanthera, leadership or fiduciary role in NetSarc and French Sarcoma Group. JLB declares no competing interests. HG declares no competing interests. ELS reports participation on a Data Safety Monitoring Board or Advisory Board for Daiichi Sankyo and Deciphera. ZB declares no competing interests. EG reports honoraria and medical writing support from Daiichi Sankyo. GS reports research funding to his institution (LUMC) from Daiichi Sankyo outside the submitted work. PL, EB, and XY are employees for Daiichi Sankyo. MAJvdS reports research funding to his institution from Daiichi Sankyo and Carbofix; consulting fees to institution from AmMax; participation on Data Safety Monitoring Board or Advisory Board and consulting fees for emectuzumab; and leadership or fiduciary role in EMSOS, Dutch Sarcoma Group (DSG), and Euro Ewing Consortium.
Figures

Similar articles
-
Tenosynovial Giant Cell Tumor Observational Platform Project (TOPP) Registry: A 2-Year Analysis of Patient-Reported Outcomes and Treatment Strategies.Oncologist. 2023 Jun 2;28(6):e425-e435. doi: 10.1093/oncolo/oyad011. Oncologist. 2023. PMID: 36869793 Free PMC article.
-
The diffuse-type tenosynovial giant cell tumor (dt-TGCT) patient journey: a prospective multicenter study.Orphanet J Rare Dis. 2021 Apr 29;16(1):191. doi: 10.1186/s13023-021-01820-6. Orphanet J Rare Dis. 2021. PMID: 33926503 Free PMC article.
-
Distinct extra-articular invasion patterns of diffuse pigmented villonodular synovitis/tenosynovial giant cell tumor in the knee joints.Knee Surg Sports Traumatol Arthrosc. 2018 Nov;26(11):3508-3514. doi: 10.1007/s00167-018-4942-2. Epub 2018 Apr 10. Knee Surg Sports Traumatol Arthrosc. 2018. PMID: 29637236
-
Treatment updates on tenosynovial giant cell tumor.Curr Opin Oncol. 2022 Jul 1;34(4):322-327. doi: 10.1097/CCO.0000000000000853. Curr Opin Oncol. 2022. PMID: 35837703 Review.
-
Pexidartinib for the treatment of adult symptomatic patients with tenosynovial giant cell tumors.Expert Rev Clin Pharmacol. 2020 Jun;13(6):571-576. doi: 10.1080/17512433.2020.1771179. Epub 2020 Jun 1. Expert Rev Clin Pharmacol. 2020. PMID: 32478598 Review.
Cited by
-
Diffuse-Type Tenosynovial Giant Cell Tumor of the Knee: Clinical Course After Anterior Open Synovectomy.Curr Oncol. 2025 Jun 11;32(6):342. doi: 10.3390/curroncol32060342. Curr Oncol. 2025. PMID: 40558285 Free PMC article.
-
Unlocking the Power of Benchmarking: Real-World-Time Data Analysis for Enhanced Sarcoma Patient Outcomes.Cancers (Basel). 2023 Sep 2;15(17):4395. doi: 10.3390/cancers15174395. Cancers (Basel). 2023. PMID: 37686671 Free PMC article.
-
Tenosynovial Giant Cell Tumor Observational Platform Project (TOPP) Registry: A 2-Year Analysis of Patient-Reported Outcomes and Treatment Strategies.Oncologist. 2023 Jun 2;28(6):e425-e435. doi: 10.1093/oncolo/oyad011. Oncologist. 2023. PMID: 36869793 Free PMC article.
References
-
- Ottaviani S, Ayral X, Dougados M, Gossec L. Pigmented villonodular synovitis: a retrospective single‐center study of 122 cases and review of the literature. Semin Arthritis Rheum. 2011;40(6):539‐546. - PubMed
-
- Staals EL, Ferrari S, Donati DM, Palmerini E. Diffuse‐type tenosynovial giant cell tumour: Current treatment concepts and future perspectives. Eur J Cancer (Oxford, England: 1990). 63, 2016:34‐40. - PubMed
-
- de Saint Aubain Somerhausen N, van de Rijn M. Tenosynovial giant cell tumour, localized type. In: Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 5, 4th ed. International Agencey for Research on Cancer (IARC); 2013:100‐101.

