Tolerance and Heavy Metal Accumulation Characteristics of Sasa argenteostriata (Regel) E.G. Camus under Zinc Single Stress and Combined Lead-Zinc Stress
- PMID: 36006129
- PMCID: PMC9415762
- DOI: 10.3390/toxics10080450
Tolerance and Heavy Metal Accumulation Characteristics of Sasa argenteostriata (Regel) E.G. Camus under Zinc Single Stress and Combined Lead-Zinc Stress
Abstract
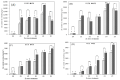
Sasa argenteostriata (Regel) E.G. Camus is a gramineous plant with the potential for phytoremediation. In this study, we aimed to determine its tolerance to zinc stress and combined lead-zinc stress and the effect of zinc on its absorption and accumulation characteristics of lead. The results showed that S. argenteostriata had good tolerance to zinc stress, and S. argenteostriata was not significantly damaged when the zinc stress concentration was 600 mg/L. Under both zinc stress and combined lead-zinc stress, the root was the main organ that accumulated heavy metals in S. argenteostriata. The presence of zinc promoted the absorption of lead by the root of S. argenteostriata, and the lead content in the root under PZ1, PZ2, PZ3 and PZ4 treatments was 2.15, 4.31, 4.47 and 6.01 times that of PZ0 on the 20 days. In the combined lead-zinc stress treatments, the toxicity of heavy metals to S. argenteostriata was mainly caused by lead. Under high concentrations of combined lead-zinc stress (PZ4), the proportion of zinc in the leaf of S. argenteostriata on the 20 days increased, which was used as a tolerance strategy to alleviate the toxicity of lead.
Keywords: Sasa argenteostriata (Regel) E.G. Camus; accumulation characteristics; combined zinc–lead stress.
Conflict of interest statement
The authors report that there are no competing interests to declare.
Figures






References
-
- Dong L.J., Tong X.J., Li X.B., Zhou J., Wang S.F., Liu B. Some developments and new insights of environmental problems and deep mining strategy for cleaner production in mines. J. Clean. Prod. 2019;210:1562–1578. doi: 10.1016/j.jclepro.2018.10.291. - DOI
-
- Obiora S.C., Chukwu A., Toteu S.F., Davies T.C. Assessment of heavy metal contamination in soils around lead (Pb)-zinc (Zn) mining areas in Enyigba, southeastern Nigeria. J. Geol. Soc. India. 2016;87:453–462. doi: 10.1007/s12594-016-0413-x. - DOI
-
- Barac N., Skrivanj S., Mutic J., Manojlovic D., Bukumiric Z., Zivojinovic D., Petrovic R., Corac A. Heavy Metals Fractionation in Agricultural Soils of Pb/Zn Mining Region and Their Transfer to Selected Vegetables. Water Air Soil Pollut. 2016;227:481. doi: 10.1007/s11270-016-3177-4. - DOI
-
- Dong J., Yang Q.W., Sun L.N., Zeng Q., Liu S.J., Pan J., Liu X.L. Assessing the concentration and potential dietary risk of heavy metals in vegetables at a Pb/Zn mine site, China. Environ. Earth Sci. 2011;64:1317–1321. doi: 10.1007/s12665-011-0992-1. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

